PKCθ signaling is required for myoblast fusion by regulating the expression of caveolin-3 and β1D integrin upstream focal adhesion kinase
- PMID: 21346196
- PMCID: PMC3078083
- DOI: 10.1091/mbc.E10-10-0821
PKCθ signaling is required for myoblast fusion by regulating the expression of caveolin-3 and β1D integrin upstream focal adhesion kinase
Abstract
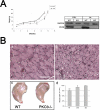
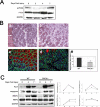
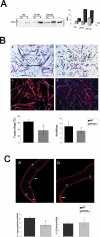
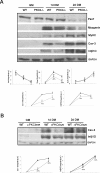
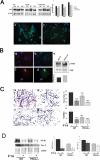
Fusion of mononucleated myoblasts to form multinucleated myofibers is an essential phase of skeletal myogenesis, which occurs during muscle development as well as during postnatal life for muscle growth, turnover, and regeneration. Many cell adhesion proteins, including integrins, have been shown to be important for myoblast fusion in vertebrates, and recently focal adhesion kinase (FAK), has been proposed as a key mediator of myoblast fusion. Here we focused on the possible role of PKC, the PKC isoform predominantly expressed in skeletal muscle, in myoblast fusion. We found that the expression of PKC is strongly up-regulated following freeze injury-induced muscle regeneration, as well as during in vitro differentiation of satellite cells (SCs; the muscle stem cells). Using both PKC knockout and muscle-specific PKC dominant-negative mutant mouse models, we observed delayed body and muscle fiber growth during the first weeks of postnatal life, when compared with wild-type (WT) mice. We also found that myofiber formation, during muscle regeneration after freeze injury, was markedly impaired in PKC mutant mice, as compared with WT. This phenotype was associated with reduced expression of the myogenic differentiation program executor, myogenin, but not with that of the SC marker Pax7. Indeed in vitro differentiation of primary muscle-derived SCs from PKC mutants resulted in the formation of thinner myotubes with reduced numbers of myonuclei and reduced fusion rate, when compared with WT cells. These effects were associated to reduced expression of the profusion genes caveolin-3 and β1D integrin and to reduced activation/phosphorylation of their up-stream regulator FAK. Indeed the exogenous expression of a constitutively active mutant form of PKC in muscle cells induced FAK phosphorylation. Moreover pharmacologically mediated full inhibition of FAK activity led to similar fusion defects in both WT and PKC-null myoblasts. We thus propose that PKC signaling regulates myoblast fusion by regulating, at least in part, FAK activity, essential for profusion gene expression.
Figures
Similar articles
-
Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion.Mol Biol Cell. 2009 Jul;20(14):3422-35. doi: 10.1091/mbc.e09-02-0175. Epub 2009 May 20. Mol Biol Cell. 2009. PMID: 19458188 Free PMC article.
-
Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA.Am J Physiol Cell Physiol. 2007 Jun;292(6):C2057-69. doi: 10.1152/ajpcell.00493.2006. Epub 2007 Jan 31. Am J Physiol Cell Physiol. 2007. PMID: 17267546
-
Suppression of protein kinase C theta contributes to enhanced myogenesis in vitro via IRS1 and ERK1/2 phosphorylation.BMC Cell Biol. 2013 Sep 21;14:39. doi: 10.1186/1471-2121-14-39. BMC Cell Biol. 2013. PMID: 24053798 Free PMC article.
-
Mechanisms regulating myoblast fusion: A multilevel interplay.Semin Cell Dev Biol. 2020 Aug;104:81-92. doi: 10.1016/j.semcdb.2020.02.004. Epub 2020 Feb 13. Semin Cell Dev Biol. 2020. PMID: 32063453 Review.
-
Signaling mechanisms in mammalian myoblast fusion.Sci Signal. 2013 Apr 23;6(272):re2. doi: 10.1126/scisignal.2003832. Sci Signal. 2013. PMID: 23612709 Free PMC article. Review.
Cited by
-
Normal muscle regeneration requires tight control of muscle cell fusion by tetraspanins CD9 and CD81.Nat Commun. 2013;4:1674. doi: 10.1038/ncomms2675. Nat Commun. 2013. PMID: 23575678
-
Targeting PKCθ Promotes Satellite Cell Self-Renewal.Int J Mol Sci. 2020 Mar 31;21(7):2419. doi: 10.3390/ijms21072419. Int J Mol Sci. 2020. PMID: 32244482 Free PMC article.
-
Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury.Int J Mol Sci. 2020 Jan 31;21(3):932. doi: 10.3390/ijms21030932. Int J Mol Sci. 2020. PMID: 32023816 Free PMC article.
-
Loss of myogenic potential and fusion capacity of muscle stem cells isolated from contractured muscle in children with cerebral palsy.Am J Physiol Cell Physiol. 2018 Aug 1;315(2):C247-C257. doi: 10.1152/ajpcell.00351.2017. Epub 2018 Apr 25. Am J Physiol Cell Physiol. 2018. PMID: 29694232 Free PMC article.
-
A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies.Int J Mol Sci. 2020 Nov 19;21(22):8736. doi: 10.3390/ijms21228736. Int J Mol Sci. 2020. PMID: 33228026 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

