Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy
- PMID: 21346220
- PMCID: PMC3269770
- DOI: 10.1212/WNL.0b013e318212a89f
Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy
Abstract
Objectives: New data suggest that glioma stem-like cells (GSCs) and neural stem/progenitor cells (NSCs) may share common origins. GSCs drive tumor proliferation and appear to be resistant to classic chemotherapy, while the effects of chemotherapy on NSCs are not well studied. As the role of NSCs in learning and memory is increasingly recognized, we need to identify drugs that reduce neurotoxicity but are still effective against glial tumors.
Methods: We treated 3 human NSC cultures and multiple low- and high-grade GSC cultures with the commonly used agents temozolomide (TMZ) and cisplatin (CIS), and with 2 newer, promising drugs: the proteasome inhibitor bortezomib (BTZ) and the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (ERL). We measured cell survival, proliferation, cell death induction, and drug resistance markers.
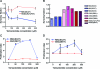
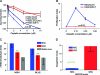
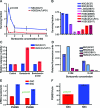
Results: TMZ decreased NSC viability, while minimally affecting GSCs. TMZ induced NSC death, which was partially compensated for by increased proliferation. CIS had similar effects. The NSC's sensitivity to TMZ and CIS correlated with low expression of the multidrug resistance gene ABCG2, but not of MGMT or MSH1/MLH2. BTZ caused an 80%decrease in GSCs, while minimally affecting NSCs. GSCs had lower proteasome levels and activity after BTZ treatment. ERL treatment also decreased GSC numbers, but not NSC viability, which correlated with low EGFR expression in NSCs compared to GSCs.
Conclusions: Newer chemotherapy agents ERL and BTZ are effective against GSCs yet produce minimal effects on NSCs, while the older drugs TMZ and CIS are more toxic for NSCs than for GSCs. The identification and testing of more selective drugs is clearly warranted.
Figures


Comment in
-
Is brain the new bone marrow? Stem cells, cancer, and cognition: a working hypothesis.Neurology. 2011 Mar 29;76(13):1118-20. doi: 10.1212/WNL.0b013e318212a928. Epub 2011 Feb 23. Neurology. 2011. PMID: 21346222 No abstract available.
-
Fighting brain tumors while protecting the brain: the stem cell story.Neurology. 2011 Mar 29;76(13):e69-70. doi: 10.1212/WNL.0b013e318215b914. Neurology. 2011. PMID: 21444897 No abstract available.
-
Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy.Neurology. 2011 Nov 29;77(22):e135; author reply e135-6. doi: 10.1212/WNL.0b013e318239ba7c. Neurology. 2011. PMID: 22123788 No abstract available.
References
-
- 2010 CBTRUS Statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. In: Central Brain Tumor Registry of the United States. Available at: http://www.cbtrus.org/reports/reports.html. Accessed March 2010
-
- Schmidinger M, Linzmayer L, Becherer A, et al. Psychometric and quality-of-life assessment in long-term glioblastoma survivors. J Neurooncol 2003;63:55–61 - PubMed
-
- Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol 1990;28:818–822 - PubMed
-
- Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 2002;20:485–493 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
