Hepcidin and iron regulation, 10 years later
- PMID: 21346250
- PMCID: PMC3099567
- DOI: 10.1182/blood-2011-01-258467
Hepcidin and iron regulation, 10 years later
Abstract
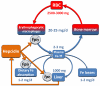
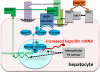
Under evolutionary pressure to counter the toxicity of iron and to maintain adequate iron supply for hemoglobin synthesis and essential metabolic functions, humans and other vertebrates have effective mechanisms to conserve iron and to regulate its concentration, storage, and distribution in tissues. The iron-regulatory hormone hepcidin, first described 10 years ago, and its receptor and iron channel ferroportin control the dietary absorption, storage, and tissue distribution of iron. Hepcidin causes ferroportin internalization and degradation, thereby decreasing iron transfer into blood plasma from the duodenum, from macrophages involved in recycling senescent erythrocytes, and from iron-storing hepatocytes. Hepcidin is feedback regulated by iron concentrations in plasma and the liver and by erythropoietic demand for iron. Genetic malfunctions affecting the hepcidin-ferroportin axis are a main cause of iron overload disorders but can also cause iron-restricted anemias. Modulation of hepcidin and ferroportin expression during infection and inflammation couples iron metabolism to host defense and decreases iron availability to invading pathogens. This response also restricts the iron supply to erythropoietic precursors and may cause or contribute to the anemia associated with infections and inflammatory disorders.
Figures
References
-
- Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. - PubMed
-
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. - PubMed
-
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

