CREB and the CRTC co-activators: sensors for hormonal and metabolic signals
- PMID: 21346730
- PMCID: PMC4324555
- DOI: 10.1038/nrm3072
CREB and the CRTC co-activators: sensors for hormonal and metabolic signals
Abstract
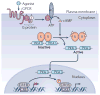
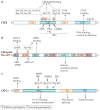
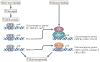
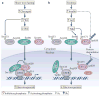
The cyclic AMP-responsive element-binding protein (CREB) is phosphorylated in response to a wide variety of signals, yet target gene transcription is only increased in a subset of cases. Recent studies indicate that CREB functions in concert with a family of latent cytoplasmic co-activators called cAMP-regulated transcriptional co-activators (CRTCs), which are activated through dephosphorylation. A dual requirement for CREB phosphorylation and CRTC dephosphorylation is likely to explain how these activator-co-activator cognates discriminate between different stimuli. Following their activation, CREB and CRTCs mediate the effects of fasting and feeding signals on the expression of metabolic programmes in insulin-sensitive tissues.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at Serine 133. Cell. 1989;59:675–680. - PubMed
-
- Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. Characterizes the role of CBP as a CREB co-activator. - PubMed
-
- Kwok R, et al. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. - PubMed
-
- Arias J, et al. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

