Dendritic spine pathology in neuropsychiatric disorders
- PMID: 21346746
- PMCID: PMC3530413
- DOI: 10.1038/nn.2741
Dendritic spine pathology in neuropsychiatric disorders
Abstract
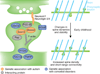
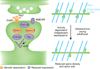
Substantial progress has been made toward understanding the genetic architecture, cellular substrates, brain circuits and endophenotypic profiles of neuropsychiatric disorders, including autism spectrum disorders (ASD), schizophrenia and Alzheimer's disease. Recent evidence implicates spiny synapses as important substrates of pathogenesis in these disorders. Although synaptic perturbations are not the only alterations relevant for these diseases, understanding the molecular underpinnings of spine pathology may provide insight into their etiologies and may reveal new drug targets. Here we discuss recent neuropathological, genetic, molecular and animal model studies that implicate structural alterations at spiny synapses in the pathogenesis of major neurological disorders, focusing on ASD, schizophrenia and Alzheimer's disease as representatives of these categories across different ages of onset. We stress the importance of reverse translation, collaborative and multidisciplinary approaches, and the study of the spatio-temporal roles of disease molecules in the context of synaptic regulatory pathways and neuronal circuits that underlie disease endophenotypes.
Figures


References
-
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. - PubMed
-
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. - PubMed
-
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. - PubMed
-
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. - PubMed
-
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

