TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier
- PMID: 21346775
- PMCID: PMC8609659
- DOI: 10.1038/jid.2011.24
TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier
Erratum in
- J Invest Dermatol. 2011 Jun;131(6):1388. Guttman, Emma [corrected to Guttman-Yassky, Emma]
Abstract
Filaggrin (FLG), loricrin (LOR), and involucrin are important epidermal barrier proteins. As psoriasis is characterized by overexpression of tumor necrosis factor-α (TNF-α) and impaired skin barrier, we investigated the expression of skin barrier proteins in psoriasis patients and whether their expression was modulated by TNF-α. The expression of FLG and LOR was found to be decreased in lesional and non-lesional skin of psoriasis patients. A correlation was found between the expression of TNF-α and epidermal barrier proteins in psoriasis. TNF-α was found to modulate the expression of FLG and LOR via a c-Jun N-terminal kinase-dependent pathway. Importantly, we report that clinical treatment of psoriasis patients with a TNF-α antagonist results in significant enhancement of epidermal barrier protein expression. Our current study suggests that TNF inhibits barrier protein expression, and TNF-α antagonists may contribute to clinical improvement in patients with psoriasis by improving barrier protein expression.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflict of interest.
Figures
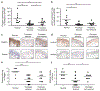




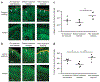
References
-
- Albanesi C, Fairchild HR, Madonna S et al. (2007) IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol 179:984–92 - PubMed
-
- Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6:328–40 - PubMed
-
- Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40 - PubMed
-
- Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M et al. (2010) Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 131:677–87 - PubMed
-
- Christophers E, Henseler T (1987) Contrasting disease patterns in psoriasis and atopic dermatitis. Arch Dermatol Res 279(Suppl):S48–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

