Human stiff-person syndrome IgG induces anxious behavior in rats
- PMID: 21346811
- PMCID: PMC3035624
- DOI: 10.1371/journal.pone.0016775
Human stiff-person syndrome IgG induces anxious behavior in rats
Abstract
Background: Anxiety is a heterogeneous behavioral domain playing a role in a variety of neuropsychiatric diseases. While anxiety is the cardinal symptom in disorders such as panic disorder, co-morbid anxious behavior can occur in a variety of diseases. Stiff person syndrome (SPS) is a CNS disorder characterized by increased muscle tone and prominent agoraphobia and anxiety. Most patients have high-titer antibodies against glutamate decarboxylase (GAD) 65. The pathogenic role of these autoantibodies is unclear.
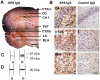
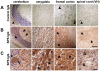
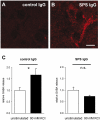
Methodology/principal findings: We re-investigated a 53 year old woman with SPS and profound anxiety for GABA-A receptor binding in the amygdala with (11)C-flumazenil PET scan and studied the potential pathogenic role of purified IgG from her plasma filtrates containing high-titer antibodies against GAD 65. We passively transferred the IgG fraction intrathecally into rats and analyzed the effects using behavioral and in vivo electrophysiological methods. In cell culture, we measured the effect of patient IgG on GABA release from hippocampal neurons. Repetitive intrathecal application of purified patient IgG in rats resulted in an anxious phenotype resembling the core symptoms of the patient. Patient IgG selectively bound to rat amygdala, hippocampus, and frontal cortical areas. In cultured rat hippocampal neurons, patient IgG inhibited GABA release. In line with these experimental results, the GABA-A receptor binding potential was reduced in the patient's amygdala/hippocampus complex. No motor abnormalities were found in recipient rats.
Conclusion/significance: The observations in rats after passive transfer lead us to propose that anxiety-like behavior can be induced in rats by passive transfer of IgG from a SPS patient positive for anti-GAD 65 antibodies. Anxiety, in this case, thus may be an antibody-mediated phenomenon with consecutive disturbance of GABAergic signaling in the amygdala region.
Conflict of interest statement
Figures


Similar articles
-
Human Stiff person syndrome IgG-containing high-titer anti-GAD65 autoantibodies induce motor dysfunction in rats.Exp Neurol. 2013 Jan;239:202-9. doi: 10.1016/j.expneurol.2012.10.013. Epub 2012 Oct 23. Exp Neurol. 2013. PMID: 23099416
-
Neuronal surface and glutamic acid decarboxylase autoantibodies in Nonparaneoplastic stiff person syndrome.JAMA Neurol. 2013 Sep 1;70(9):1140-9. doi: 10.1001/jamaneurol.2013.3499. JAMA Neurol. 2013. PMID: 23877118 Free PMC article.
-
Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies.Neurology. 2001 Sep 11;57(5):780-4. doi: 10.1212/wnl.57.5.780. Neurology. 2001. PMID: 11552003
-
Stiff-person syndrome (SPS) and anti-GAD-related CNS degenerations: protean additions to the autoimmune central neuropathies.J Autoimmun. 2011 Sep;37(2):79-87. doi: 10.1016/j.jaut.2011.05.005. Epub 2011 Jun 16. J Autoimmun. 2011. PMID: 21680149 Review.
-
Stiff-person Syndrome and GAD Antibody-spectrum Disorders: GABAergic Neuronal Excitability, Immunopathogenesis and Update on Antibody Therapies.Neurotherapeutics. 2022 Apr;19(3):832-847. doi: 10.1007/s13311-022-01188-w. Epub 2022 Jan 27. Neurotherapeutics. 2022. PMID: 35084720 Free PMC article. Review.
Cited by
-
Interactions of Human Autoantibodies with Hippocampal GABAergic Synaptic Transmission - Analyzing Antibody-Induced Effects ex vivo.Front Neurol. 2015 Jun 11;6:136. doi: 10.3389/fneur.2015.00136. eCollection 2015. Front Neurol. 2015. PMID: 26124746 Free PMC article.
-
An update on malignant tumor-related stiff person syndrome spectrum disorders: clinical mechanism, treatment, and outcomes.Front Neurol. 2023 Oct 4;14:1209302. doi: 10.3389/fneur.2023.1209302. eCollection 2023. Front Neurol. 2023. PMID: 37859648 Free PMC article. Review.
-
Pseudoagoraphobia, a Diagnostic Clue in Stiff-Limb Syndrome.Mov Disord Clin Pract. 2020 Mar 5;7(3):313-317. doi: 10.1002/mdc3.12911. eCollection 2020 Apr. Mov Disord Clin Pract. 2020. PMID: 32258231 Free PMC article.
-
Pathophysiological Effects of Autoantibodies in Autoimmune Encephalitides.Cells. 2023 Dec 20;13(1):15. doi: 10.3390/cells13010015. Cells. 2023. PMID: 38201219 Free PMC article. Review.
-
Gender differences in associations of glutamate decarboxylase 1 gene (GAD1) variants with panic disorder.PLoS One. 2012;7(5):e37651. doi: 10.1371/journal.pone.0037651. Epub 2012 May 25. PLoS One. 2012. PMID: 22662185 Free PMC article.
References
-
- Meinck HM, Ricker K, Hulser PJ, Schmid E, Peiffer J, et al. Stiff man syndrome: clinical and laboratory findings in eight patients. J Neurol. 1994;241:157–166. - PubMed
-
- Dalakas MC, Fujii M, Li M, McElroy B. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology. 2000;55:1531–1535. - PubMed
-
- Meinck HM, Thompson PD. Stiff man syndrome and related conditions. Mov Disord. 2002;17:853–866. - PubMed
-
- Floeter MK, Valls-Sole J, Toro C, Jacobowitz D, Hallett M. Physiologic studies of spinal inhibitory circuits in patients with stiff-person syndrome. Neurology. 1998;51:85–93. - PubMed

