Timescales of multineuronal activity patterns reflect temporal structure of visual stimuli
- PMID: 21346812
- PMCID: PMC3035626
- DOI: 10.1371/journal.pone.0016758
Timescales of multineuronal activity patterns reflect temporal structure of visual stimuli
Abstract
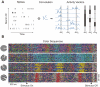
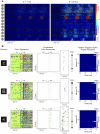
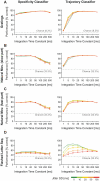
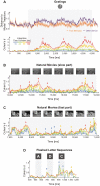
The investigation of distributed coding across multiple neurons in the cortex remains to this date a challenge. Our current understanding of collective encoding of information and the relevant timescales is still limited. Most results are restricted to disparate timescales, focused on either very fast, e.g., spike-synchrony, or slow timescales, e.g., firing rate. Here, we investigated systematically multineuronal activity patterns evolving on different timescales, spanning the whole range from spike-synchrony to mean firing rate. Using multi-electrode recordings from cat visual cortex, we show that cortical responses can be described as trajectories in a high-dimensional pattern space. Patterns evolve on a continuum of coexisting timescales that strongly relate to the temporal properties of stimuli. Timescales consistent with the time constants of neuronal membranes and fast synaptic transmission (5-20 ms) play a particularly salient role in encoding a large amount of stimulus-related information. Thus, to faithfully encode the properties of visual stimuli the brain engages multiple neurons into activity patterns evolving on multiple timescales.
Conflict of interest statement
Figures

References
-
- Perkel D, Bullock TH. Neural coding. Neurosci Res Prog Bulletin. 1968;6:221–348.
-
- Eggermont JJ. Is there a neural code? Neurosci Biobehav Rev. 1998;22:355–370. - PubMed
-
- Adrian ED. New York: W.W. Norton & Co; 1928. The basis of sensation: the action of the sense organs.
-
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65, 111-25. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

