Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease
- PMID: 21347238
- PMCID: PMC3039660
- DOI: 10.1371/journal.pone.0016959
Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease
Abstract
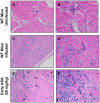
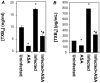
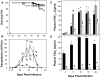
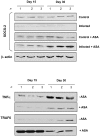
Chagas disease, caused by infection with Trypanosoma cruzi, is an important cause of cardiovascular disease. It is increasingly clear that parasite-derived prostaglandins potently modulate host response and disease progression. Here, we report that treatment of experimental T. cruzi infection (Brazil strain) beginning 5 days post infection (dpi) with aspirin (ASA) increased mortality (2-fold) and parasitemia (12-fold). However, there were no differences regarding histopathology or cardiac structure or function. Delayed treatment with ASA (20 mg/kg) beginning 60 dpi did not increase parasitemia or mortality but improved ejection fraction. ASA treatment diminished the profile of parasite- and host-derived circulating prostaglandins in infected mice. To distinguish the effects of ASA on the parasite and host bio-synthetic pathways we infected cyclooxygenase-1 (COX-1) null mice with the Brazil-strain of T. cruzi. Infected COX-1 null mice displayed a reduction in circulating levels of thromboxane (TX)A(2) and prostaglandin (PG)F(2α). Parasitemia was increased in COX-1 null mice compared with parasitemia and mortality in ASA-treated infected mice indicating the effects of ASA on mortality potentially had little to do with inhibition of prostaglandin metabolism. Expression of SOCS-2 was enhanced, and TRAF6 and TNFα reduced, in the spleens of infected ASA-treated mice. Ablation of the initial innate response to infection may cause the increased mortality in ASA-treated mice as the host likely succumbs more quickly without the initiation of the "cytokine storm" during acute infection. We conclude that ASA, through both COX inhibition and other "off-target" effects, modulates the progression of acute and chronic Chagas disease. Thus, eicosanoids present during acute infection may act as immunomodulators aiding the transition to and maintenance of the chronic phase of the disease. A deeper understanding of the mechanism of ASA action may provide clues to the differences between host response in the acute and chronic T. cruzi infection.
Conflict of interest statement
Figures


Similar articles
-
Protective effect of aspirin treatment on mouse behavior in the acute phase of experimental infection with Trypanosoma cruzi.Parasitol Res. 2018 Jan;117(1):189-200. doi: 10.1007/s00436-017-5693-6. Epub 2017 Dec 1. Parasitol Res. 2018. PMID: 29196837
-
Protective role of acetylsalicylic acid in experimental Trypanosoma cruzi infection: evidence of a 15-epi-lipoxin A₄-mediated effect.PLoS Negl Trop Dis. 2013 Apr 18;7(4):e2173. doi: 10.1371/journal.pntd.0002173. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23638194 Free PMC article.
-
Bioactive lipids in Trypanosoma cruzi infection.Adv Parasitol. 2011;76:1-31. doi: 10.1016/B978-0-12-385895-5.00001-3. Adv Parasitol. 2011. PMID: 21884885 Free PMC article. Review.
-
Aspirin prevents atrophy of esophageal nitrergic myenteric neurons in a mouse model of chronic Chagas disease.Dis Esophagus. 2017 Feb 1;30(2):1-8. doi: 10.1111/dote.12449. Dis Esophagus. 2017. PMID: 26725535
-
Nonsteroidal Anti-Inflammatory Drugs and Experimental Chagas Disease: An Unsolved Question.Parasite Immunol. 2024 Jul;46(7):e13057. doi: 10.1111/pim.13057. Parasite Immunol. 2024. PMID: 39008292 Review.
Cited by
-
Specialized Proresolving Mediators in Innate and Adaptive Immune Responses in Airway Diseases.Physiol Rev. 2018 Jul 1;98(3):1335-1370. doi: 10.1152/physrev.00026.2017. Physiol Rev. 2018. PMID: 29717929 Free PMC article. Review.
-
Protective effect of aspirin treatment on mouse behavior in the acute phase of experimental infection with Trypanosoma cruzi.Parasitol Res. 2018 Jan;117(1):189-200. doi: 10.1007/s00436-017-5693-6. Epub 2017 Dec 1. Parasitol Res. 2018. PMID: 29196837
-
Identification of a functional prostanoid-like receptor in the protozoan parasite, Trypanosoma cruzi.Parasitol Res. 2013 Apr;112(4):1417-25. doi: 10.1007/s00436-012-3271-5. Epub 2013 Feb 13. Parasitol Res. 2013. PMID: 23403991 Free PMC article.
-
Protective role of acetylsalicylic acid in experimental Trypanosoma cruzi infection: evidence of a 15-epi-lipoxin A₄-mediated effect.PLoS Negl Trop Dis. 2013 Apr 18;7(4):e2173. doi: 10.1371/journal.pntd.0002173. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23638194 Free PMC article.
-
Bioactive lipids in Trypanosoma cruzi infection.Adv Parasitol. 2011;76:1-31. doi: 10.1016/B978-0-12-385895-5.00001-3. Adv Parasitol. 2011. PMID: 21884885 Free PMC article. Review.
References
-
- Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. - PubMed
-
- Huang H, Chan J, Wittner M, Weiss LM, Bacchi CJ, et al. Trypanosoma cruzi induces myocardial nitric oxide synthase. Cardiovasc Pathol. 1997;6:161–166. - PubMed
-
- Petkova SB, Huang H, Factor SM, Pestell RG, Bouzahzah B, et al. The role of endothelin in the pathogenesis of Chagas' disease. Int J Parasitol. 2001;31:499–511. - PubMed
-
- Petkova SB, Tanowitz HB, Magazine HI, Factor SM, Chan J, et al. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc Pathol. 2000;9:257–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

