Unusual structures are present in DNA fragments containing super-long Huntingtin CAG repeats
- PMID: 21347256
- PMCID: PMC3037965
- DOI: 10.1371/journal.pone.0017119
Unusual structures are present in DNA fragments containing super-long Huntingtin CAG repeats
Abstract
Background: In the R6/2 mouse model of Huntington's disease (HD), expansion of the CAG trinucleotide repeat length beyond about 300 repeats induces a novel phenotype associated with a reduction in transcription of the transgene.
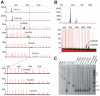
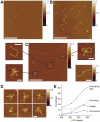
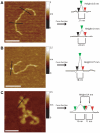
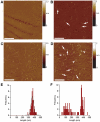
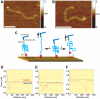
Methodology/principal findings: We analysed the structure of polymerase chain reaction (PCR)-generated DNA containing up to 585 CAG repeats using atomic force microscopy (AFM). As the number of CAG repeats increased, an increasing proportion of the DNA molecules exhibited unusual structural features, including convolutions and multiple protrusions. At least some of these features are hairpin loops, as judged by cross-sectional analysis and sensitivity to cleavage by mung bean nuclease. Single-molecule force measurements showed that the convoluted DNA was very resistant to untangling. In vitro replication by PCR was markedly reduced, and TseI restriction enzyme digestion was also hindered by the abnormal DNA structures. However, significantly, the DNA gained sensitivity to cleavage by the Type III restriction-modification enzyme, EcoP15I.
Conclusions/significance: "Super-long" CAG repeats are found in a number of neurological diseases and may also appear through CAG repeat instability. We suggest that unusual DNA structures associated with super-long CAG repeats decrease transcriptional efficiency in vitro. We also raise the possibility that if these structures occur in vivo, they may play a role in the aetiology of CAG repeat diseases such as HD.
Conflict of interest statement
Figures


References
-
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. - PubMed
-
- López Castel A, Cleary JD, Pearson CE. Repeat instability as the basis for human diseases and as a potential target for therapy. Nat Rev Mol Cell Biol. 2010;11:165–170. - PubMed
-
- Andresen JM, Gayán J, Djousse L, Roberts S, Brocklebank D, et al. The relationship between CAG repeat length and age of onset differs for Huntington's disease patients with juvenile onset or adult onset. Ann Hum Genet. 2007;71:295–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

