Pyramidal cells in prefrontal cortex of primates: marked differences in neuronal structure among species
- PMID: 21347276
- PMCID: PMC3039119
- DOI: 10.3389/fnana.2011.00002
Pyramidal cells in prefrontal cortex of primates: marked differences in neuronal structure among species
Abstract
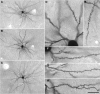
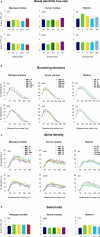
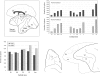
The most ubiquitous neuron in the cerebral cortex, the pyramidal cell, is characterized by markedly different dendritic structure among different cortical areas. The complex pyramidal cell phenotype in granular prefrontal cortex (gPFC) of higher primates endows specific biophysical properties and patterns of connectivity, which differ from those in other cortical regions. However, within the gPFC, data have been sampled from only a select few cortical areas. The gPFC of species such as human and macaque monkey includes more than 10 cortical areas. It remains unknown as to what degree pyramidal cell structure may vary among these cortical areas. Here we undertook a survey of pyramidal cells in the dorsolateral, medial, and orbital gPFC of cercopithecid primates. We found marked heterogeneity in pyramidal cell structure within and between these regions. Moreover, trends for gradients in neuronal complexity varied among species. As the structure of neurons determines their computational abilities, memory storage capacity and connectivity, we propose that these specializations in the pyramidal cell phenotype are an important determinant of species-specific executive cortical functions in primates.
Keywords: baboon; cognition; connectivity; guenon; human; macaque; primate; spine.
Figures




Similar articles
-
Specializations of the granular prefrontal cortex of primates: implications for cognitive processing.Anat Rec A Discov Mol Cell Evol Biol. 2006 Jan;288(1):26-35. doi: 10.1002/ar.a.20278. Anat Rec A Discov Mol Cell Evol Biol. 2006. PMID: 16342214
-
Pyramidal neurons of granular prefrontal cortex of the galago: complexity in evolution of the psychic cell in primates.Anat Rec A Discov Mol Cell Evol Biol. 2005 Jul;285(1):610-8. doi: 10.1002/ar.a.20198. Anat Rec A Discov Mol Cell Evol Biol. 2005. PMID: 15912521
-
Specialization in pyramidal cell structure in the sensory-motor cortex of the Chacma baboon (Papio ursinus) with comparative notes on macaque and vervet monkeys.Anat Rec A Discov Mol Cell Evol Biol. 2005 Sep;286(1):854-65. doi: 10.1002/ar.a.20217. Anat Rec A Discov Mol Cell Evol Biol. 2005. PMID: 16100710
-
Pyramidal cell development: postnatal spinogenesis, dendritic growth, axon growth, and electrophysiology.Front Neuroanat. 2014 Aug 12;8:78. doi: 10.3389/fnana.2014.00078. eCollection 2014. Front Neuroanat. 2014. PMID: 25161611 Free PMC article. Review.
-
Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function.Cereb Cortex. 2003 Nov;13(11):1124-38. doi: 10.1093/cercor/bhg093. Cereb Cortex. 2003. PMID: 14576205 Review.
Cited by
-
Maintenance of delay-period activity in working memory task is modulated by local network structure.PLoS Comput Biol. 2024 Sep 3;20(9):e1012415. doi: 10.1371/journal.pcbi.1012415. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39226309 Free PMC article.
-
Transcriptomic cytoarchitecture reveals principles of human neocortex organization.Science. 2023 Oct 13;382(6667):eadf6812. doi: 10.1126/science.adf6812. Epub 2023 Oct 13. Science. 2023. PMID: 37824655 Free PMC article.
-
Human and chimpanzee shared and divergent neurobiological systems for general and specific cognitive brain functions.Proc Natl Acad Sci U S A. 2023 May 30;120(22):e2218565120. doi: 10.1073/pnas.2218565120. Epub 2023 May 22. Proc Natl Acad Sci U S A. 2023. PMID: 37216540 Free PMC article.
-
Circuit dynamics of superficial and deep CA1 pyramidal cells and inhibitory cells in freely-moving macaques.bioRxiv [Preprint]. 2024 May 22:2023.12.06.570369. doi: 10.1101/2023.12.06.570369. bioRxiv. 2024. Update in: Cell Rep. 2024 Aug 27;43(8):114519. doi: 10.1016/j.celrep.2024.114519. PMID: 38106053 Free PMC article. Updated. Preprint.
-
Quantitative assessment of prefrontal cortex in humans relative to nonhuman primates.Proc Natl Acad Sci U S A. 2018 May 29;115(22):E5183-E5192. doi: 10.1073/pnas.1721653115. Epub 2018 May 8. Proc Natl Acad Sci U S A. 2018. PMID: 29739891 Free PMC article.
References
-
- Ashford J. W., Fuster J. M. (1985). Occipital and inferotemporal responses to visual signals in the monkey. Exp. Neurol. 90, 444–446 - PubMed
-
- Ballesteros-Yánez I., Muñoz A., Contreras J., Gonzalez J., Rodriguez-Veiga E., DeFelipe J. (2005). The double bouquet cell in the human cerebral cortex and a comparison with other mammals. J. Comp. Neurol. 486, 344–360 - PubMed
-
- Barbas H. (1992). Architecture and cortical connections of the prefrontal cortex in the rhesus monkey. Adv. Neurol. 57, 91–115 - PubMed
-
- Barbas H. (2000). Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 52, 319–330 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

