RNA binding protein CUGBP2/CELF2 mediates curcumin-induced mitotic catastrophe of pancreatic cancer cells
- PMID: 21347286
- PMCID: PMC3037932
- DOI: 10.1371/journal.pone.0016958
RNA binding protein CUGBP2/CELF2 mediates curcumin-induced mitotic catastrophe of pancreatic cancer cells
Abstract
Background: Curcumin inhibits the growth of pancreatic cancer tumor xenografts in nude mice; however, the mechanism of action is not well understood. It is becoming increasingly clear that RNA binding proteins regulate posttranscriptional gene expression and play a critical role in RNA stability and translation. Here, we have determined that curcumin modulates the expression of RNA binding protein CUGBP2 to inhibit pancreatic cancer growth.
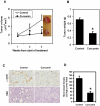
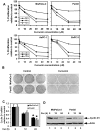
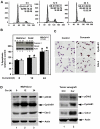
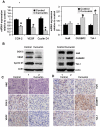
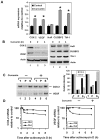
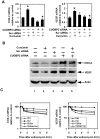
Methodology/principal findings: In this study, we show that curcumin treated tumor xenografts have a significant reduction in tumor volume and angiogenesis. Curcumin inhibited the proliferation, while inducing G2-M arrest and apoptosis resulting in mitotic catastrophe of various pancreatic cancer cells. This was further confirmed by increased phosphorylation of checkpoint kinase 2 (Chk2) protein coupled with higher levels of nuclear cyclin B1 and Cdc-2. Curcumin increased the expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) mRNA, but protein levels were lower. Furthermore, curcumin increased the expression of RNA binding proteins CUGBP2/CELF2 and TIA-1. CUGBP2 binding to COX-2 and VEGF mRNA was also enhanced, thereby increasing mRNA stability, the half-life changing from 30 min to 8 h. On the other hand, silencer-mediated knockdown of CUGBP2 partially restored the expression of COX-2 and VEGF even with curcumin treatment. COX-2 and VEGF mRNA levels were reduced to control levels, while proteins levels were higher.
Conclusion/significance: Curcumin inhibits pancreatic tumor growth through mitotic catastrophe by increasing the expression of RNA binding protein CUGBP2, thereby inhibiting the translation of COX-2 and VEGF mRNA. These data suggest that translation inhibition is a novel mechanism of action for curcumin during the therapeutic intervention of pancreatic cancers.
Conflict of interest statement
Figures
Similar articles
-
Novel intestinal splice variants of RNA-binding protein CUGBP2: isoform-specific effects on mitotic catastrophe.Am J Physiol Gastrointest Liver Physiol. 2008 Apr;294(4):G971-81. doi: 10.1152/ajpgi.00540.2007. Epub 2008 Feb 7. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18258790 Free PMC article.
-
Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2.Am J Physiol Gastrointest Liver Physiol. 2008 Apr;294(4):G1025-32. doi: 10.1152/ajpgi.00602.2007. Epub 2008 Feb 21. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18292181
-
CUGBP2 downregulation by prostaglandin E2 protects colon cancer cells from radiation-induced mitotic catastrophe.Am J Physiol Gastrointest Liver Physiol. 2008 May;294(5):G1235-44. doi: 10.1152/ajpgi.00037.2008. Epub 2008 Mar 6. Am J Physiol Gastrointest Liver Physiol. 2008. Retraction in: Am J Physiol Gastrointest Liver Physiol. 2025 Aug 1;329(2):G328. doi: 10.1152/ajpgi.00037.2008_RET. PMID: 18325984 Retracted.
-
Clinical effects of curcumin in enhancing cancer therapy: A systematic review.BMC Cancer. 2020 Aug 24;20(1):791. doi: 10.1186/s12885-020-07256-8. BMC Cancer. 2020. PMID: 32838749 Free PMC article.
-
The biological and therapeutic relevance of mRNA translation in cancer.Nat Rev Clin Oncol. 2011 May;8(5):280-91. doi: 10.1038/nrclinonc.2011.16. Epub 2011 Mar 1. Nat Rev Clin Oncol. 2011. PMID: 21364523 Review.
Cited by
-
CELFish ways to modulate mRNA decay.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):695-707. doi: 10.1016/j.bbagrm.2013.01.001. Epub 2013 Jan 15. Biochim Biophys Acta. 2013. PMID: 23328451 Free PMC article. Review.
-
LncRNA CRNDE facilitates epigenetic suppression of CELF2 and LATS2 to promote proliferation, migration and chemoresistance in hepatocellular carcinoma.Cell Death Dis. 2020 Aug 11;11(8):676. doi: 10.1038/s41419-020-02853-8. Cell Death Dis. 2020. PMID: 32826865 Free PMC article.
-
Pleotropic role of RNA binding protein CELF2 in autophagy induction.Mol Carcinog. 2019 Aug;58(8):1400-1409. doi: 10.1002/mc.23023. Epub 2019 Apr 24. Mol Carcinog. 2019. PMID: 31020708 Free PMC article.
-
Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells.Oncol Lett. 2018 May;15(5):6777-6783. doi: 10.3892/ol.2018.8112. Epub 2018 Feb 23. Oncol Lett. 2018. PMID: 29616136 Free PMC article.
-
Long Non-Coding RNAs and RNA-Binding Proteins in Pancreatic Cancer Development and Progression.Cancers (Basel). 2025 May 8;17(10):1601. doi: 10.3390/cancers17101601. Cancers (Basel). 2025. PMID: 40427100 Free PMC article. Review.
References
-
- Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008;13:562–576. - PubMed
-
- Real FX. A “catastrophic hypothesis” for pancreas cancer progression. Gastroenterology. 2003;124:1958–1964. - PubMed
-
- Duffy JP, Reber HA. Pancreatic neoplasms. Curr Opin Gastroenterol. 2003;19:458–466. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(Suppl 1):S18–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

