Evidence for mesenchymal-epithelial transition associated with mouse hepatic stem cell differentiation
- PMID: 21347296
- PMCID: PMC3037942
- DOI: 10.1371/journal.pone.0017092
Evidence for mesenchymal-epithelial transition associated with mouse hepatic stem cell differentiation
Abstract
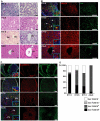
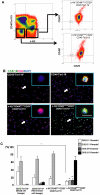
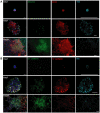
Mesenchymal-epithelial transition events are related to embryonic development, tissue construction, and wound healing. Stem cells are involved in all of these processes, at least in part. However, the direct evidence of mesenchymal-epithelial transition associated with stem cells is unclear. To determine whether mesenchymal-epithelial transition occurs in liver development and/or the differentiation process of hepatic stem cells in vitro, we analyzed a variety of murine liver tissues from embryonic day 11.5 to adults and the colonies derived from hepatic stem/progenitor cells isolated with flow cytometry. The results of gene expression, immunohistochemistry and Western blot showed that as liver develops, the expression of epithelial markers such as Cytokeratin18 and E-cadherin increase, while expression of mesenchymal markers such as vimentin and N-cadherin decreased. On the other hand, in freshly isolated hepatic stem cells, the majority of cells (65.0%) co-express epithelial and mesenchymal markers; this proportion is significantly higher than observed in hematopoietic cells, non-hematopoietic cells and non-stem cell fractions. Likewise, in stem cell-derived colonies cultured over time, upregulation of epithelial genes (Cytokeratin-18 and E-cadherin) occurred simultaneously with downregulation of mesenchymal genes (vimentin and Snail1). Furthermore, in the fetal liver, vimentin-positive cells in the non-hematopoietic fraction had distinct proliferative activity and expressed early the hepatic lineage marker alpha-fetoprotein.
Conclusion: Hepatic stem cells co-express mesenchymal and epithelial markers; the mesenchymal-epithelial transition occurred in both liver development and differentiation of hepatic stem/progenitor cells in vitro. Besides as a mesenchymal marker, vimentin is a novel indicator for cell proliferative activity and undifferentiated status in liver cells.
Conflict of interest statement
Figures
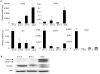


Similar articles
-
Evidence for epithelial-mesenchymal transition in adult human pancreatic exocrine cells.J Histochem Cytochem. 2010 Sep;58(9):807-23. doi: 10.1369/jhc.2010.955807. Epub 2010 Jun 7. J Histochem Cytochem. 2010. PMID: 20530463 Free PMC article.
-
The epithelial-mesenchymal transition promotes transdifferentiation of subcutaneously implanted hepatic oval cells into mesenchymal tumor tissue.Stem Cells Dev. 2009 Nov;18(9):1293-8. doi: 10.1089/scd.2008.0321. Stem Cells Dev. 2009. PMID: 19226223 Free PMC article.
-
N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays.Hum Reprod. 2017 Nov 1;32(11):2254-2268. doi: 10.1093/humrep/dex289. Hum Reprod. 2017. PMID: 29040564
-
Epithelial-mesenchymal transition in Rhesus monkey embryonic stem cell colonies: a model for processes involved in gastrulation?Cells Tissues Organs. 2007;185(1-3):48-50. doi: 10.1159/000101302. Cells Tissues Organs. 2007. PMID: 17587807 Review.
-
Epithelial-to-mesenchymal transitions in the liver.Hepatology. 2009 Dec;50(6):2007-13. doi: 10.1002/hep.23196. Hepatology. 2009. PMID: 19824076 Free PMC article. Review.
Cited by
-
Epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions in the colon.World J Gastroenterol. 2012 Feb 21;18(7):601-8. doi: 10.3748/wjg.v18.i7.601. World J Gastroenterol. 2012. PMID: 22363130 Free PMC article. Review.
-
Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation.Stem Cell Res Ther. 2013 Jun 24;4(3):75. doi: 10.1186/scrt226. Stem Cell Res Ther. 2013. PMID: 23800436 Free PMC article.
-
Stem cell niches for skin regeneration.Int J Biomater. 2012;2012:926059. doi: 10.1155/2012/926059. Epub 2012 Jun 3. Int J Biomater. 2012. PMID: 22701121 Free PMC article.
-
New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer.Nat Rev Mol Cell Biol. 2019 Feb;20(2):69-84. doi: 10.1038/s41580-018-0080-4. Nat Rev Mol Cell Biol. 2019. PMID: 30459476 Review.
-
MiR-302a Regenerates Human Corneal Endothelial Cells against IFN-γ-Induced Cell Death.Cells. 2022 Dec 22;12(1):36. doi: 10.3390/cells12010036. Cells. 2022. PMID: 36611829 Free PMC article.
References
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. - PubMed
-
- Polo JM, Hochedlinger K. When Fibroblasts MET iPSCs. Cell Stem Cell. 2010;7:5–6. - PubMed
-
- Li R, Liang J, Ni S, Zhou T, Qing X, et al. A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts. Cell Stem Cell. 2010;7:51–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

