Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice
- PMID: 21347299
- PMCID: PMC3037945
- DOI: 10.1371/journal.pone.0016861
Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice
Abstract
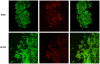
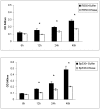
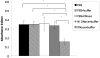
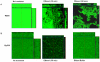
Bacteria form complex and highly elaborate surface adherent communities known as biofilms which are held together by a self-produced extracellular matrix. We have previously shown that by adopting a biofilm mode of existence in vivo, the gram negative bacterial pathogens Bordetella bronchiseptica and Bordetella pertussis are able to efficiently colonize and persist in the mammalian respiratory tract. In general, the bacterial biofilm matrix includes polysaccharides, proteins and extracellular DNA (eDNA). In this report, we investigated the function of DNA in Bordetella biofilm development. We show that DNA is a significant component of Bordetella biofilm matrix. Addition of DNase I at the initiation of biofilm growth inhibited biofilm formation. Treatment of pre-established mature biofilms formed under both static and flow conditions with DNase I led to a disruption of the biofilm biomass. We next investigated whether eDNA played a role in biofilms formed in the mouse respiratory tract. DNase I treatment of nasal biofilms caused considerable dissolution of the biofilm biomass. In conclusion, these results suggest that eDNA is a crucial structural matrix component of both in vitro and in vivo formed Bordetella biofilms. This is the first evidence for the ability of DNase I to disrupt bacterial biofilms formed on host organs.
Conflict of interest statement
Figures



Similar articles
-
The Bordetella Bps polysaccharide is critical for biofilm development in the mouse respiratory tract.J Bacteriol. 2007 Nov;189(22):8270-6. doi: 10.1128/JB.00785-07. Epub 2007 Jun 22. J Bacteriol. 2007. PMID: 17586629 Free PMC article.
-
Bordetella biofilms: a lifestyle leading to persistent infections.Pathog Dis. 2016 Feb;74(1):ftv108. doi: 10.1093/femspd/ftv108. Epub 2015 Nov 19. Pathog Dis. 2016. PMID: 26586694 Free PMC article. Review.
-
Interplay of virulence factors and signaling molecules: albumin and calcium-mediated biofilm regulation in Bordetella bronchiseptica.J Bacteriol. 2025 Apr 17;207(4):e0044524. doi: 10.1128/jb.00445-24. Epub 2025 Mar 26. J Bacteriol. 2025. PMID: 40135913 Free PMC article.
-
The BvgAS signal transduction system regulates biofilm development in Bordetella.J Bacteriol. 2005 Feb;187(4):1474-84. doi: 10.1128/JB.187.4.1474-1484.2005. J Bacteriol. 2005. PMID: 15687212 Free PMC article.
-
Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens.FEMS Immunol Med Microbiol. 2003 Jul 15;37(2-3):121-8. doi: 10.1016/S0928-8244(03)00068-3. FEMS Immunol Med Microbiol. 2003. PMID: 12832115 Review.
Cited by
-
Continuous nondestructive monitoring of Bordetella pertussis biofilms by Fourier transform infrared spectroscopy and other corroborative techniques.Anal Bioanal Chem. 2007 Mar;387(5):1759-67. doi: 10.1007/s00216-006-1079-9. Epub 2007 Jan 10. Anal Bioanal Chem. 2007. PMID: 17216159
-
Earthworm symbiont Verminephrobacter eiseniae mediates natural transformation within host egg capsules using type IV pili.Front Microbiol. 2014 Oct 29;5:546. doi: 10.3389/fmicb.2014.00546. eCollection 2014. Front Microbiol. 2014. PMID: 25400622 Free PMC article.
-
BpsR modulates Bordetella biofilm formation by negatively regulating the expression of the Bps polysaccharide.J Bacteriol. 2012 Jan;194(2):233-42. doi: 10.1128/JB.06020-11. Epub 2011 Nov 4. J Bacteriol. 2012. PMID: 22056934 Free PMC article.
-
Stenotrophomonas maltophilia: an emerging global opportunistic pathogen.Clin Microbiol Rev. 2012 Jan;25(1):2-41. doi: 10.1128/CMR.00019-11. Clin Microbiol Rev. 2012. PMID: 22232370 Free PMC article. Review.
-
Effect of DNase Treatment on Adhesion and Early Biofilm Formation of Enterococcus Faecalis.Eur Endod J. 2018 Jul 19;3(2):82-86. doi: 10.14744/eej.2018.55264. eCollection 2018. Eur Endod J. 2018. PMID: 32161861 Free PMC article.
References
-
- Sebaihia M, Preston A, Maskell DJ, Kuzmiak H, Connell TD, et al. Comparison of the genome sequence of the poultry pathogen Bordetella avium with those of B. bronchiseptica, B. pertussis, and B. parapertussis reveals extensive diversity in surface structures associated with host interaction. J Bacteriol. 2006;188:6002–6015. - PMC - PubMed
-
- Bemis DA. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet Clin North Am Small Anim Pract. 1992;22:1173–1186. - PubMed
-
- Mooi FR. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect Genet Evol. 10:36–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

