New insights into the apoptotic process in mollusks: characterization of caspase genes in Mytilus galloprovincialis
- PMID: 21347300
- PMCID: PMC3037946
- DOI: 10.1371/journal.pone.0017003
New insights into the apoptotic process in mollusks: characterization of caspase genes in Mytilus galloprovincialis
Abstract
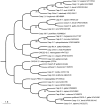
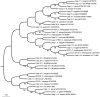
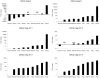
Apoptosis is an essential biological process in the development and maintenance of immune system homeostasis. Caspase proteins constitute the core of the apoptotic machinery and can be categorized as either initiators or effectors of apoptosis. Although the genes encoding caspase proteins have been described in vertebrates and in almost all invertebrate phyla, there are few reports describing the initiator and executioner caspases or the modulation of their expression by different stimuli in different apoptotic pathways in bivalves. In the present work, we characterized two initiator and four executioner caspases in the mussel Mytilus galloprovincialis. Both initiators and executioners showed structural features that make them different from other caspase proteins already described. Evaluation of the genes' tissue expression patterns revealed extremely high expression levels within the gland and gills, where the apoptotic process is highly active due to the clearance of damaged cells. Hemocytes also showed high expression values, probably due to of the role of apoptosis in the defense against pathogens. To understand the mechanisms of caspase gene regulation, hemocytes were treated with UV-light, environmental pollutants and pathogen-associated molecular patterns (PAMPs) and apoptosis was evaluated by microscopy, flow cytometry and qPCR techniques. Our results suggest that the apoptotic process could be tightly regulated in bivalve mollusks by overexpression/suppression of caspase genes; additionally, there is evidence of caspase-specific responses to pathogens and pollutants. The apoptotic process in mollusks has a similar complexity to that of vertebrates, but presents unique features that may be related to recurrent exposure to environmental changes, pollutants and pathogens imposed by their sedentary nature.
Conflict of interest statement
Figures




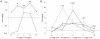


Similar articles
-
Histopathological and apoptotic changes on marine mussels Mytilus galloprovincialis (Lamark, 1819) following exposure to environmental pollutants.Mar Pollut Bull. 2016 Aug 15;109(1):184-191. doi: 10.1016/j.marpolbul.2016.05.084. Epub 2016 Jun 11. Mar Pollut Bull. 2016. PMID: 27301687
-
Genes of the mitochondrial apoptotic pathway in Mytilus galloprovincialis.PLoS One. 2013 Apr 23;8(4):e61502. doi: 10.1371/journal.pone.0061502. Print 2013. PLoS One. 2013. PMID: 23626691 Free PMC article.
-
Phylogenetic analysis of the caspase family in bivalves: implications for programmed cell death, immune response and development.BMC Genomics. 2021 Jan 25;22(1):80. doi: 10.1186/s12864-021-07380-0. BMC Genomics. 2021. PMID: 33494703 Free PMC article.
-
Expansion and diversity of caspases in Mytilus coruscus contribute to larval metamorphosis and environmental adaptation.BMC Genomics. 2024 Mar 27;25(1):314. doi: 10.1186/s12864-024-10238-w. BMC Genomics. 2024. PMID: 38532358 Free PMC article. Review.
-
The complexity of apoptotic cell death in mollusks: An update.Fish Shellfish Immunol. 2015 Sep;46(1):79-87. doi: 10.1016/j.fsi.2015.03.038. Epub 2015 Apr 9. Fish Shellfish Immunol. 2015. PMID: 25862972 Review.
Cited by
-
In vitro Evaluation of Programmed Cell Death in the Immune System of Pacific Oyster Crassostrea gigas by the Effect of Marine Toxins.Front Immunol. 2021 Apr 1;12:634497. doi: 10.3389/fimmu.2021.634497. eCollection 2021. Front Immunol. 2021. PMID: 33868255 Free PMC article.
-
The effects of cold stress on Mytilus species in the natural environment.Cell Stress Chaperones. 2020 Nov;25(6):821-832. doi: 10.1007/s12192-020-01109-w. Epub 2020 Apr 15. Cell Stress Chaperones. 2020. PMID: 32297161 Free PMC article. Review.
-
Sequencing, De Novo Assembly, and Annotation of the Transcriptome of the Endangered Freshwater Pearl Bivalve, Cristaria plicata, Provides Novel Insights into Functional Genes and Marker Discovery.PLoS One. 2016 Feb 12;11(2):e0148622. doi: 10.1371/journal.pone.0148622. eCollection 2016. PLoS One. 2016. PMID: 26872384 Free PMC article.
-
Transcriptomic Analysis of Differentially Expressed Genes During Larval Development of Rapana venosa by Digital Gene Expression Profiling.G3 (Bethesda). 2016 Jul 7;6(7):2181-93. doi: 10.1534/g3.116.029314. G3 (Bethesda). 2016. PMID: 27194808 Free PMC article.
-
Transcriptomic Analysis Reveals Insights on Male Infertility in Octopus maya Under Chronic Thermal Stress.Front Physiol. 2019 Jan 15;9:1920. doi: 10.3389/fphys.2018.01920. eCollection 2018. Front Physiol. 2019. PMID: 30697164 Free PMC article.
References
-
- Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. - PubMed
-
- Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nature Immunol. 2003;4:410–415. - PubMed
-
- Koyama AH, Adachi A, Irie H. Physiological significance of apoptosis during animal virus infection. Int Rev Immunol. 2003;22:341–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

