The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex
- PMID: 21347354
- PMCID: PMC3037364
- DOI: 10.1371/journal.ppat.1001282
The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex
Abstract
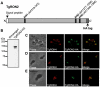
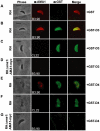
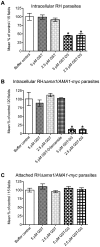
Host cell invasion by apicomplexan parasites requires formation of the moving junction (MJ), a ring-like apposition between the parasite and host plasma membranes that the parasite migrates through during entry. The Toxoplasma MJ is a secreted complex including TgAMA1, a transmembrane protein on the parasite surface, and a complex of rhoptry neck proteins (TgRON2/4/5/8) described as host cell-associated. How these proteins connect the parasite and host cell has not previously been described. Here we show that TgRON2 localizes to the MJ and that two short segments flanking a hydrophobic stretch near its C-terminus (D3 and D4) independently associate with the ectodomain of TgAMA1. Pre-incubation of parasites with D3 (fused to glutathione S-transferase) dramatically reduces invasion but does not prevent injection of rhoptry bulb proteins. Hence, the entire C-terminal region of TgRON2 forms the crucial bridge between TgAMA1 and the rest of the MJ complex but this association is not required for rhoptry protein injection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

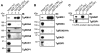
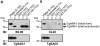
References
-
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
-
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. - PubMed
-
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. - PubMed
-
- Michel R, Schupp K, Raether W, Bierther FW. Formation of a close junction during invasion of erythrocytes by Toxoplasma gondii in vitro. Int J Parasitol. 1980;10:309–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

