Behaviour of telomere and telomerase during aging and regeneration in zebrafish
- PMID: 21347393
- PMCID: PMC3036734
- DOI: 10.1371/journal.pone.0016955
Behaviour of telomere and telomerase during aging and regeneration in zebrafish
Abstract
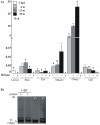
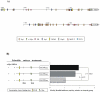
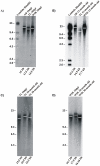
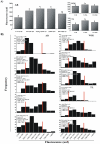
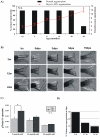
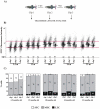
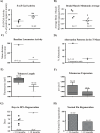
Telomere length and telomerase activity are important factors in the pathobiology of human diseases. Age-related diseases and premature aging syndromes are characterized by short telomeres, which can compromise cell viability, whereas tumour cells can prevent telomere loss by aberrantly upregulating telomerase. The zebrafish (Danio rerio) offers multiple experimental manipulation advantages over other vertebrate models and, therefore, it has been recently considered as a potential model for aging, cancer, and regeneration studies. However, it has only partially been exploited to shed light on these fundamental biological processes. The aim of this study was, therefore, to investigate telomere length and telomerase expression and activity in different strains of zebrafish obtained from different stock centres to determine whether they undergo any changes during aging and regeneration. We found that although both telomerase expression and telomere length increased from embryo to adulthood stages, they drastically declined in aged fish despite telomerase activity was detected in different tissues of old fish. In addition, we observed a weaker upregulation of telomerase expression in regenerating fins of old fish, which well correlates with their impaired regeneration capacity. Strikingly, telomeres were elongated or maintained during the fin regeneration process at all ages and after repeated amputations, likely to support high cell proliferation rates. We conclude that the expression of telomerase and telomere length are closely related during the entire life cycle of the fish and that these two parameters can be used as biomarkers of aging in zebrafish. Our results also reveal a direct relationship between the expression of telomerase, telomere length and the efficiency of tissue regeneration.
Conflict of interest statement
Figures

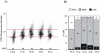
References
-
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nature Genet. 2007;8:353–367. - PubMed
-
- Feitsma H, Cuppen E. Zebrafish as a cancer model. Mo.l Cancer Res. 2008;6:685–694. - PubMed
-
- Gerhard GS, Cheng KC. A call to fins! Zebrafish as a gerontological model. Aging Cell. 2002;1:104–111. - PubMed
-
- Gerhard GS. Small laboratory fish as models for aging research. Ageing Res Rev. 2007 May; 2007;6(1):64–72. - PubMed
-
- Kishi S, Uchiyama J, Baughman AM, Goto T, Lin MC, et al. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp Gerontol. 2003;38:777–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

