Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves
- PMID: 21347406
- PMCID: PMC3036584
- DOI: 10.1371/journal.pone.0016295
Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves
Abstract
Background: The maintenance of the intestinal epithelium is of great importance for the survival of the organism. A possible nervous control of epithelial cell renewal was studied in rats and mice.
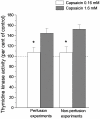
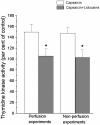
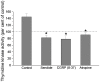
Methods: Mucosal afferent nerves were stimulated by exposing the intestinal mucosa to capsaicin (1.6 mM), which stimulates intestinal external axons. Epithelial cell renewal was investigated in the jejunum by measuring intestinal thymidine kinase (TK) activity, intestinal (3)H-thymidine incorporation into DNA, and the number of crypt cells labeled with BrdU. The influence of the external gut innervation was minimized by severing the periarterial nerves.
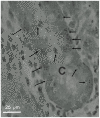
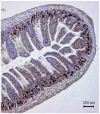
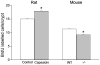
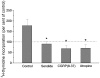
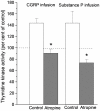
Principal findings: Luminal capsaicin increased all the studied variables, an effect nervously mediated to judge from inhibitory effects on TK activity or (3)H-thymidine incorporation into DNA by exposing the mucosa to lidocaine (a local anesthetic) or by giving four different neurotransmitter receptor antagonists i.v. (muscarinic, nicotinic, neurokinin1 (NK1) or calcitonin gene related peptide (CGRP) receptors). After degeneration of the intestinal external nerves capsaicin did not increase TK activity, suggesting the involvement of an axon reflex. Intra-arterial infusion of Substance P (SP) or CGRP increased intestinal TK activity, a response abolished by muscarinic receptor blockade. Immunohistochemistry suggested presence of M3 and M5 muscarinic receptors on the intestinal stem/progenitor cells. We propose that the stem/progenitor cells are controlled by cholinergic nerves, which, in turn, are influenced by mucosal afferent neuron(s) releasing acetylcholine and/or SP and/or CGRP. In mice lacking the capsaicin receptor, thymidine incorporation into DNA and number of crypt cells labeled with BrdU was lower than in wild type animals suggesting that nerves are important also in the absence of luminal capsaicin, a conclusion also supported by the observation that atropine lowered thymidine incorporation into DNA by 60% in control rat segments.
Conclusion: Enteric nerves are of importance in maintaining the intestinal epithelial barrier.
Conflict of interest statement
Figures



References
-
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. - PubMed
-
- Furness JB. Oxford: Blackwell Publishing Ltd; 2006. The enteric nervous system.
-
- Vidrich A, Buzan JM, De La Rue SA, Cohn SA. Physiology of gastrointestinal stem cells. In Physiology of the Gastrointestinal Tract Fourth edition, In: Johnson LR, editor. Elsevier Inc., Amsterdam; 2006. pp. 307–344.
-
- Tutton PJ. The influence of cholinoceptor activity on the mitotic rate in the crypts of Lieberkűhn in rat jejunum. Clin Exp Pharmacol Physiol. 1975;2:269–76. - PubMed
-
- Tutton PJ, Barkla DH. Neural control of colonic cell proliferation. Cancer. 1980;45:1172–1177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

