A coproantigen diagnostic test for Strongyloides infection
- PMID: 21347447
- PMCID: PMC3035667
- DOI: 10.1371/journal.pntd.0000955
A coproantigen diagnostic test for Strongyloides infection
Abstract
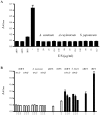
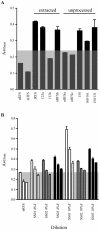
Accurate diagnosis of infection with the parasite Strongyloides stercoralis is hampered by the low concentration of larvae in stool, rendering parasitological diagnosis insensitive. Even if the more sensitive agar plate culture method is used repeated stool sampling is necessary to achieve satisfactory sensitivity. In this manuscript we describe the development of a coproantigen ELISA for diagnosis of infection. Polyclonal rabbit antiserum was raised against Strongyloides ratti excretory/secretory (E/S) antigen and utilized to develop an antigen capture ELISA. The assay enabled detection of subpatent rodent S. ratti and human S. stercoralis infection. No cross-reactivity was observed with purified E/S from Schistosoma japonicum, the hookworms Ancylostoma caninum, A. ceylanicum, nor with fecal samples collected from rodents harboring Trichuris muris or S. mansoni infection. Strongyloides coproantigens that appear stable when frozen as formalin-extracted fecal supernatants stored at -20 °C remained positive up to 270 days of storage, whereas supernatants stored at 4 °C tested negative. These results indicate that diagnosis of human strongyloidiasis by detection of coproantigen is an approach worthy of further development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Sato Y, Otsuru M, Takara M, Shiroma Y. Intradermal reactions in strongyloidiasis. Int J Parasitol. 1986;16:87–91. - PubMed
-
- Page WA, Dempsey K, McCarthy JS. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans R Soc Trop Med Hyg. 2006;100:1056–1062. - PubMed
-
- Conway DJ, Atkins NS, Lillywhite JE, Bailey JW, Robinson RD, et al. Immunodiagnosis of Strongyloides stercoralis infection: a method for increasing the specificity of the indirect ELISA. Trans R Soc Trop Med Hyg. 1993;87:173–176. - PubMed
-
- Allan JC, Craig PS. Coproantigens in gut tapeworm infections: Hymenolepis diminuta in rats. Parasitol Res. 1989;76:68–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

