2,4-Diaminopyrimidines as potent inhibitors of Trypanosoma brucei and identification of molecular targets by a chemical proteomics approach
- PMID: 21347454
- PMCID: PMC3035674
- DOI: 10.1371/journal.pntd.0000956
2,4-Diaminopyrimidines as potent inhibitors of Trypanosoma brucei and identification of molecular targets by a chemical proteomics approach
Abstract
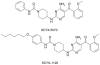
Background: There is an urgent need to develop new, safe and effective treatments for human African trypanosomiasis (HAT) because current drugs have extremely poor safety profiles and are difficult to administer. Here we report the discovery of 2,4-diaminopyrimidines, exemplified by 4-[4-amino-5-(2-methoxy-benzoyl)-pyrimidin-2-ylamino]-piperidine-1-carboxylic acid phenylamide (SCYX-5070), as potent inhibitors of Trypanosoma brucei and the related trypanosomatid protozoans Leishmania spp.
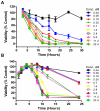
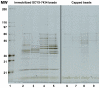
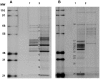
Methodology/principal findings: In this work we show that loss of T. brucei viability following SCYX-5070 exposure was dependent on compound concentration and incubation time. Pulse incubation of T. brucei with SCYX-5070 demonstrates that a short period of exposure (10-12 hrs) is required to produce irreversible effects on survival or commit the parasites to death. SCYX-5070 cured an acute trypanosomiasis infection in mice without exhibiting signs of compound related acute or chronic toxicity. To identify the molecular target(s) responsible for the mechanism of action of 2,4-diaminopyrimidines against trypanosomatid protozoa, a representative analogue was immobilized on a solid matrix (sepharose) and used to isolate target proteins from parasite extracts. Mitogen-activated protein kinases (MAPKs) and cdc2-related kinases (CRKs) were identified as the major proteins specifically bound to the immobilized compound, suggesting their participation in the pharmacological effects of 2,4-diaminopyrimidines against trypanosomatid protozoan parasites.
Conclusions/significance: Results show that 2,4-diaminopyrimidines have a good in vitro and in vivo pharmacological profile against trypanosomatid protozoans and that MAPKs and CRKs are potential molecular targets of these compounds. The 2,4-diminipyrimidines may serve as suitable leads for the development of novel treatments for HAT.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis.PLoS Negl Trop Dis. 2011 Jun;5(6):e1151. doi: 10.1371/journal.pntd.0001151. Epub 2011 Jun 28. PLoS Negl Trop Dis. 2011. PMID: 21738803 Free PMC article.
-
The use of Sulfonamide Derivatives in the Treatment of Trypanosomatid Parasites including Trypanosoma cruzi, Trypanosoma brucei, and Leishmania ssp.Med Chem. 2020;16(1):24-38. doi: 10.2174/1573406415666190620141109. Med Chem. 2020. PMID: 31218962 Review.
-
Anilinoquinoline based inhibitors of trypanosomatid proliferation.PLoS Negl Trop Dis. 2018 Nov 26;12(11):e0006834. doi: 10.1371/journal.pntd.0006834. eCollection 2018 Nov. PLoS Negl Trop Dis. 2018. PMID: 30475800 Free PMC article.
-
Treatment of African trypanosomiasis with cordycepin and adenosine deaminase inhibitors in a mouse model.J Infect Dis. 2005 Nov 1;192(9):1658-65. doi: 10.1086/496896. Epub 2005 Sep 30. J Infect Dis. 2005. PMID: 16206083
-
Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis.Curr Top Med Chem. 2011;11(16):2060-71. doi: 10.2174/156802611796575902. Curr Top Med Chem. 2011. PMID: 21619513 Free PMC article. Review.
Cited by
-
Naphthoquinone derivatives exert their antitrypanosomal activity via a multi-target mechanism.PLoS Negl Trop Dis. 2013;7(1):e2012. doi: 10.1371/journal.pntd.0002012. Epub 2013 Jan 17. PLoS Negl Trop Dis. 2013. PMID: 23350008 Free PMC article.
-
Proteomic profile response of Paracoccidioides lutzii to the antifungal argentilactone.Front Microbiol. 2015 Jun 18;6:616. doi: 10.3389/fmicb.2015.00616. eCollection 2015. Front Microbiol. 2015. PMID: 26150808 Free PMC article.
-
Advances in Development of New Treatment for Leishmaniasis.Biomed Res Int. 2015;2015:815023. doi: 10.1155/2015/815023. Epub 2015 May 11. Biomed Res Int. 2015. PMID: 26078965 Free PMC article. Review.
-
Laboratory Selection of Trypanosomatid Pathogens for Drug Resistance.Pharmaceuticals (Basel). 2022 Jan 24;15(2):135. doi: 10.3390/ph15020135. Pharmaceuticals (Basel). 2022. PMID: 35215248 Free PMC article. Review.
-
Experimental Strategies to Explore Drug Action and Resistance in Kinetoplastid Parasites.Microorganisms. 2020 Jun 24;8(6):950. doi: 10.3390/microorganisms8060950. Microorganisms. 2020. PMID: 32599761 Free PMC article. Review.
References
-
- Croft S. Pharmacological approaches to antitrypanosomal chemotherapy. Mem Inst Oswaldo Cruz, Rio de Janeiro. 1999;94:215–220. - PubMed
-
- Burri C, Keiser J. Pharmacokinetic investigations in patients from northern Angola refractory to melasoprol treatment. Trop Med Int Health. 2001;6:412–420. - PubMed
-
- Pépin J, Milord F. The treatment of human African trypanosomiasis. Adv Parasitol. 1994;33:1–47. - PubMed
-
- Brun R, Schumacher R, Schmid C, Kunz C, Burri C. The phenomenon of treatment failures in human African trypanosomosis. Trop Med Int Health. 2001;6:906–914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

