Characterization of the structure and DNA complexity of mung bean mitochondrial nucleoids
- PMID: 21347700
- PMCID: PMC3932694
- DOI: 10.1007/s10059-011-0036-4
Characterization of the structure and DNA complexity of mung bean mitochondrial nucleoids
Abstract
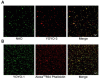
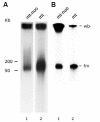
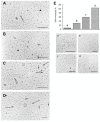
Electron microscopic images of mitochondrial nucleoids isolated from mung bean seedlings revealed a relatively homogeneous population of particles, each consisting of a chromatin-like structure associated with a membrane component. Association of F-actin with mitochondrial nucleoids was also observed. The mitochondrial nucleoid structure identified in situ showed heterogeneous genomic organization. After pulsed-field gel electrophoresis (PFGE), a large proportion of the mitochondrial nucleoid DNA remained in the well, whereas the rest migrated as a 50-200 kb smear zone. This PFGE migration pattern was not affected by high salt, topoisomerase I or latrunculin B treatments; however, the mobility of a fraction of the fast-moving DNA decreased conspicuously following an in-gel ethidium-enhanced UV-irradiation treatment, suggesting that molecules with intricately compact structures were present in the 50-200 kb region. Approximately 70% of the mitochondrial nucleoid DNA molecules examined via electron microscopy were open circles, supercoils, complex forms, and linear molecules with interspersed sigma-shaped structures and/or loops. Increased sensitivity of mtDNA to DNase I was found after mitochondrial nucleoids were pretreated with high salt. This result indicates that some loosely bound or peripheral DNA binding proteins protected the mtDNA from DNase I degradation.
Figures





Similar articles
-
Actin in mung bean mitochondria and implications for its function.Plant Cell. 2011 Oct;23(10):3727-44. doi: 10.1105/tpc.111.087403. Epub 2011 Oct 7. Plant Cell. 2011. PMID: 21984697 Free PMC article.
-
Structural and functional characterizations of mung bean mitochondrial nucleoids.Nucleic Acids Res. 2005 Aug 22;33(15):4725-39. doi: 10.1093/nar/gki783. Print 2005. Nucleic Acids Res. 2005. PMID: 16116038 Free PMC article.
-
Correlation between mtDNA complexity and mtDNA replication mode in developing cotyledon mitochondria during mung bean seed germination.New Phytol. 2017 Jan;213(2):751-763. doi: 10.1111/nph.14158. Epub 2016 Sep 9. New Phytol. 2017. PMID: 27611966
-
Evolutionary tinkering with mitochondrial nucleoids.Trends Cell Biol. 2007 Dec;17(12):586-92. doi: 10.1016/j.tcb.2007.08.007. Epub 2007 Nov 5. Trends Cell Biol. 2007. PMID: 17981466 Review.
-
Organization of DNA in Mammalian Mitochondria.Int J Mol Sci. 2019 Jun 5;20(11):2770. doi: 10.3390/ijms20112770. Int J Mol Sci. 2019. PMID: 31195723 Free PMC article. Review.
Cited by
-
The amount and integrity of mtDNA in maize decline with development.Planta. 2013 Feb;237(2):603-17. doi: 10.1007/s00425-012-1802-z. Epub 2012 Nov 16. Planta. 2013. PMID: 23229060
-
DNA maintenance in plastids and mitochondria of plants.Front Plant Sci. 2015 Oct 29;6:883. doi: 10.3389/fpls.2015.00883. eCollection 2015. Front Plant Sci. 2015. PMID: 26579143 Free PMC article. Review.
-
The alternative reality of plant mitochondrial DNA: One ring does not rule them all.PLoS Genet. 2019 Aug 30;15(8):e1008373. doi: 10.1371/journal.pgen.1008373. eCollection 2019 Aug. PLoS Genet. 2019. PMID: 31469821 Free PMC article.
References
-
- Andre C.P., Walbot V. Pulsed-field gel mapping of maize mitochondrial chromosomes. Mol. Gen. Genet. (1995);247:255–263. - PubMed
-
- Backert S., Börner T. Phage T-4-like intermediates of DNA replication and recombination in the mitochondria of the higher plant Chenopodium album (L.). Curr. Genet. (2000);37:304–314. - PubMed
-
- Backert S., Lurz R., Börner T. Electron micro-scopic investigation of mitochondrial DNA from Chenopodium album (L.). Curr. Genet. (1996);29:427–436. - PubMed
-
- Backert S., Lurz R., Oyarzabal O.A., Borner T. High content, size and distribution of single-stranded DNA in the mitochondria of Chenopodium album (L.). Plant Mol. Biol. (1997);33:1037–1050. - PubMed
-
- Bendich A.J. Structural analysis of mitochondrial DNA molecules from fungi and plants using moving pictures and pulsed-field gel electrophoresis. J. Mol. Biol. (1996);255:564–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

