Biochemical and morphological effects of hypoxic environment on human embryonic stem cells in long-term culture and differentiating embryoid bodies
- PMID: 21347709
- PMCID: PMC3932683
- DOI: 10.1007/s10059-011-0016-8
Biochemical and morphological effects of hypoxic environment on human embryonic stem cells in long-term culture and differentiating embryoid bodies
Abstract
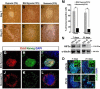
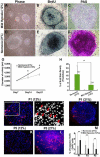
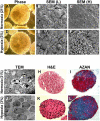
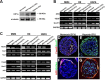
The mammalian reproductive tract is known to contain 1.5-5.3% oxygen (O(2)), but human embryonic stem cells (hESCs) derived from preimplantation embryos are typically cultured under 21% O(2) tension. The aim of this study was to investigate the effects of O(2) tension on the long-term culture of hESCs and on cell-fate determination during early differentiation. hESCs and embryoid bodies (EBs) were grown under different O(2) tensions (3, 12, and 21% O(2)). The expression of markers associated with pluripotency, embryonic germ layers, and hypoxia was analyzed using RTPCR, immunostaining, and Western blotting. Proliferation, apoptosis, and chromosomal aberrations were examined using BrdU incorporation, caspase-3 immunostaining, and karyotype analysis, respectively. Structural and morphological changes of EBs under different O(2) tensions were comparatively examined using azan- and hematoxylineosin staining, and scanning and transmission electron microscopy. Mild hypoxia (12% O(2)) increased the number of cells expressing Oct4/Nanog and reduced BrdU incorporation and aneuploidy. The percentage of cells positive for active caspase-3, which was high during normoxia (21% O(2)), gradually decreased when hESCs were continuously cultured under mild hypoxia. EBs subjected to hypoxia (3% O(2)) exhibited well-differentiated microvilli on their surface, secreted high levels of collagen, and showed enhanced differentiation into primitive endoderm. These changes were associated with increased expression of Foxa2, Sox17, AFP, and GATA4 on the EB periphery. Our data suggest that mild hypoxia facilitates the slow mitotic division of hESCs in long-term culture and reduces the frequency of chromosomal abnormalities and apoptosis. In addition, hypoxia promotes the differentiation of EBs into extraembryonic endoderm.
Figures

References
-
- Abeyta M.J., Clark A.T., Rodriguez R.T., Bodnar M.S., Pera R.A., Firpo M.T. Unique gene expression signatures of independently-derived human embryonic stem cell lines. Hum. Mol. Genet. (2004);13:601–608. - PubMed
-
- Amit M., Margulets V., Segev H., Shariki K., Laevsky I., Coleman R., Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol. Reprod. (2003);68:2150–2156. - PubMed
-
- Barberini F., Makabe S., Franchitto G., Correr S., Relucenti M., Heyn R., Familiari G. Ultrastructural dynamics of the human endometrium from 14 to 22 weeks of gestation. Arch. Histol. Cytol. (2007);70:21–28. - PubMed
-
- Bassett C.A., Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. (1961);190:460–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

