Mycobacterium tuberculosis and the intimate discourse of a chronic infection
- PMID: 21349098
- PMCID: PMC3174472
- DOI: 10.1111/j.1600-065X.2010.00984.x
Mycobacterium tuberculosis and the intimate discourse of a chronic infection
Abstract
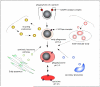
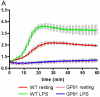
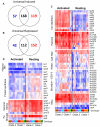
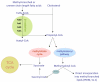
Mycobacterium tuberculosis is an extremely successful pathogen that demonstrates the capacity to modulate its host both at the cellular and tissue levels. At the cellular level, the bacterium enters its host macrophage and arrests phagosome maturation, thus avoiding many of the microbicidal responses associated with this phagocyte. Nonetheless, the intracellular environment places certain demands on the pathogen, which, in response, senses the environmental shifts and upregulates specific metabolic programs to allow access to nutrients, minimize the consequences of stress, and sustain infection. Despite its intracellular niche, Mycobacterium tuberculosis demonstrates a marked capacity to modulate the tissues surrounding infected cells through the release of potent, bioactive cell wall constituents. These cell wall lipids are released from the host cell by an exocytic process and induce physiological changes in neighboring phagocytes, which drives formation of a granuloma. This tissue response leads to the generation and accumulation of caseous debris and the progression of the human tuberculosis granuloma. Completion of the life cycle of tuberculosis requires damaging the host to release infectious bacteria into the airways to spread the infection. This damage reflects the pathogen's ability to subvert the host's innate and acquired immune responses to its own nefarious ends.
© 2011 John Wiley & Sons A/S.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

