The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility
- PMID: 21349153
- PMCID: PMC3042979
- DOI: 10.1186/2045-8118-8-5
The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility
Abstract
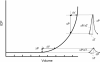
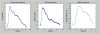
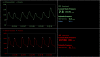
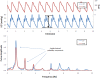
The maintenance of adequate blood flow to the brain is critical for normal brain function; cerebral blood flow, its regulation and the effect of alteration in this flow with disease have been studied extensively and are very well understood. This flow is not steady, however; the systolic increase in blood pressure over the cardiac cycle causes regular variations in blood flow into and throughout the brain that are synchronous with the heart beat. Because the brain is contained within the fixed skull, these pulsations in flow and pressure are in turn transferred into brain tissue and all of the fluids contained therein including cerebrospinal fluid. While intracranial pulsatility has not been a primary focus of the clinical community, considerable data have accrued over the last sixty years and new applications are emerging to this day. Investigators have found it a useful marker in certain diseases, particularly in hydrocephalus and traumatic brain injury where large changes in intracranial pressure and in the biomechanical properties of the brain can lead to significant changes in pressure and flow pulsatility. In this work, we review the history of intracranial pulsatility beginning with its discovery and early characterization, consider the specific technologies such as transcranial Doppler and phase contrast MRI used to assess various aspects of brain pulsations, and examine the experimental and clinical studies which have used pulsatility to better understand brain function in health and with disease.
Figures




References
-
- Sato E, Feke GT, Menke MN, Wallace McMeel J. Retinal haemodynamics in patients with age-related macular degeneration. Eye (Lond) 2006;20:697–702. - PubMed
-
- Moritani T, Crouse SF, Shea CH, Davidson N, Nakamura E. Arterial pulse wave velocity, Fourier pulsatility index, and blood lipid profiles. Med Sci Sports Exerc. 1987;19:404–409. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

