Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans
- PMID: 21349400
- PMCID: PMC3124959
- DOI: 10.1016/j.jacc.2010.09.066
Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans
Abstract
Objectives: The aim of this paper was to study mechanisms of formation of fractionated electrograms on the posterior left atrial wall (PLAW) in human paroxysmal atrial fibrillation (AF).
Background: The mechanisms responsible for complex fractionated atrial electrogram formation during AF are poorly understood.
Methods: In 24 patients, we induced sustained AF by pacing from a pulmonary vein. We analyzed transitions between organized patterns and changes in electrogram morphology leading to fractionation in relation to interbeat interval duration (systolic interval [SI]) and dominant frequency. Computer simulations of rotors helped in the interpretation of the results.
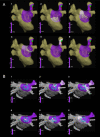
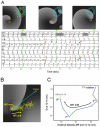
Results: Organized patterns were recorded 31 ± 18% of the time. In 47% of organized patterns, the electrograms and PLAW activation sequence were similar to those of incoming waves during pulmonary vein stimulation that induced AF. Transitions to fractionation were preceded by significant increases in electrogram duration, spike number, and SI shortening (R(2) = 0.94). Similarly, adenosine infusion during organized patterns caused significant SI shortening leading to fractionated electrograms formation. Activation maps during organization showed incoming wave patterns, with earliest activation located closest to the highest dominant frequency site. Activation maps during transitions to fragmentation showed areas of slowed conduction and unidirectional block. Simulations predicted that SI abbreviation that heralds fractionated electrograms formation might result from a Doppler effect on wave fronts preceding an approaching rotor or by acceleration of a stationary or meandering, remotely located source.
Conclusions: During induced AF, SI shortening after either drift or acceleration of a source results in intermittent fibrillatory conduction and formation of fractionated electrograms at the PLAW.
Copyright © 2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. - PubMed
-
- Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. - PubMed
-
- Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. - PubMed
-
- Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. - PubMed
-
- Di Biase L, Elayi CS, Fahmy TS, et al. Atrial Fibrillation Ablation Strategies for Paroxysmal Patients: Randomized Comparison Between Different Techniques. Circ Arrhythmia Electrophysiol. 2009;2:113–119. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

