A critical role of Gbetagamma in tumorigenesis and metastasis of breast cancer
- PMID: 21349837
- PMCID: PMC3075671
- DOI: 10.1074/jbc.M110.206615
A critical role of Gbetagamma in tumorigenesis and metastasis of breast cancer
Abstract
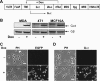
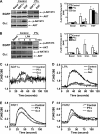
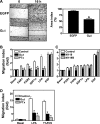
A growing body of evidence indicates that G protein-coupled receptors (GPCRs) are involved in breast tumor progression and that targeting GPCRs may be a novel adjuvant strategy in cancer treatment. However, due to the redundant role of multiple GPCRs in tumor development, it may be necessary to target a common signaling component downstream of these receptors to achieve maximum efficacy. GPCRs transmit signals through heterotrimeric G proteins composed of Gα and Gβγ subunits. Here we evaluated the role of Gβγ in breast tumor growth and metastasis both in vitro and in vivo. Our data show that blocking Gβγ signaling with Gα(t) or small molecule inhibitors blocked serum-induced breast tumor cell proliferation as well as tumor cell migration induced by various GPCRs in vitro. Moreover, induced expression of Gα(t) in MDA-MB-231 cells inhibited primary tumor formation and retarded growth of existing breast tumors in nude mice. Blocking Gβγ signaling also dramatically reduced the incidence of spontaneous lung metastasis from primary tumors and decreased tumor formation in the experimental lung metastasis model. Additional studies indicate that Gβγ signaling may also play a role in the generation of a tumor microenvironment permissive for tumor progression, because the inhibition of Gβγ signaling attenuated leukocyte infiltration and angiogenesis in primary breast tumors. Taken together, our data demonstrate a critical role of Gβγ signaling in promoting breast tumor growth and metastasis and suggest that targeting Gβγ may represent a novel therapeutic approach for breast cancer.
Figures


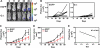
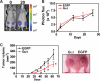



References
-
- Coughlin S. S., Ekwueme D. U. (2009) Cancer Epidemiol. 33, 315–318 - PubMed
-
- Jemal A., Siegel R., Xu J., Ward E. (2010) CA Cancer J. Clin. 60, 277–300 - PubMed
-
- Dorsam R. T., Gutkind J. S. (2007) Nat. Rev. Cancer 7, 79–94 - PubMed
-
- Schmid B. C., Rudas M., Rezniczek G. A., Leodolter S., Zeillinger R. (2004) Breast Cancer Res. Treat. 84, 247–250 - PubMed
-
- Cabioglu N., Sahin A., Doucet M., Yavuz E., Igci A. O., Yildirim E., Aktas E., Bilgic S., Kiran B., Deniz G., Price J. E. (2005) Clin. Exp. Metastasis 22, 39–46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

