Are interferon-γ release assays useful for diagnosing active tuberculosis in a high-burden setting?
- PMID: 21349910
- PMCID: PMC5463537
- DOI: 10.1183/09031936.00181610
Are interferon-γ release assays useful for diagnosing active tuberculosis in a high-burden setting?
Abstract
Although interferon-γ release assays (IGRAs) are intended for diagnosing latent tuberculosis (TB), we hypothesised that in a high-burden setting: 1) the magnitude of the response when using IGRAs can distinguish active TB from other diagnoses; 2) IGRAs may aid in the diagnosis of smear-negative TB; and 3) IGRAs could be useful as rule-out tests for active TB. We evaluated the accuracy of two IGRAs (QuantiFERON®-TB Gold In-tube (QFT-GIT) and T-SPOT®.TB) in 395 patients (27% HIV-infected) with suspected TB in Cape Town, South Africa. IGRA sensitivity and specificity (95% CI) were 76% (68-83%) and 42% (36-49%) for QFT-GIT and 84% (77-90%) and 47% (40-53%) for T-SPOT®.TB, respectively. Although interferon-γ responses were significantly higher in the TB versus non-TB groups (p<0.0001), varying the cut-offs did not improve discriminatory ability. In culture-negative patients, depending on whether those with clinically diagnosed TB were included or excluded from the analysis, the negative predictive value (NPV) of QFT-GIT, T-SPOT®.TB and chest radiograph in smear-negative patients varied between 85 and 89, 87 and 92, and 98% (for chest radiograph), respectively. Overall accuracy was independent of HIV status and CD4 count. In a high-burden setting, IGRAs alone do not have value as rule-in or -out tests for active TB. In smear-negative patients, chest radiography had better NPV even in HIV-infected patients.
Figures
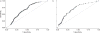
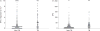

References
-
- Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
-
- WHO. Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva: World Health Organization; 2008.
-
- Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl. 1):S15–S27. - PubMed
-
- Getahun H, Harrington M, O’Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. - PubMed
-
- Burman WJ, Jones BE. Clinical and radiographic features of HIV-related tuberculosis. Semin Respir Infect. 2003;18:263–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
