Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations
- PMID: 21349926
- PMCID: PMC3168855
- DOI: 10.1378/chest.10-2865
Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations
Abstract
Background: Mutations in the human gene encoding the protein component of telomerase (TERT) are the most common genetic defect in patients with familial idiopathic pulmonary fibrosis (IPF). The subclinical phenotypes of asymptomatic members of these families have not been evaluated with respect to TERT mutation status or telomere length.
Methods: We measured a variety of pulmonary, blood, skin, and bone parameters for 20 subjects with heterozygous TERT mutations (carriers) and 20 family members who had not inherited a TERT mutation (noncarriers) to identify the spectrum of phenotypes associated with mutations in this gene. The two groups were matched for sex, age, and cigarette smoking. Three TERT mutation carriers had IPF (IPF carriers). The rest of the carriers were apparently healthy (asymptomatic carriers) and were compared with the noncarriers.
Results: Asymptomatic carriers exhibited significantly lower diffusing capacity of lung for carbon monoxide (Dlco), impaired recruitment of Dlco with exercise, radiographic signs of lung fibrosis, and increased fractional lung tissue volume quantified by high-resolution chest CT scan than noncarriers. RBC and platelet counts were significantly lower, and the mean corpuscular volume and mean corpuscular hemoglobin concentration were significantly higher in carriers than in noncarriers. Carriers reported significantly earlier graying of hair than noncarriers. TERT mutation status is more accurately predicted by short telomere lengths than any of these measured phenotypes.
Conclusions: TERT mutation carriers exhibit early preclinical signs of lung fibrosis, bone marrow dysfunction, and premature graying. These clinical features and short telomere lengths characterize patients with germline TERT mutations.
Figures

 ), TERT mutation carriers with IPF (IPF carriers, ■), and subjects without TERT mutations (noncarriers, ○). A, The telomere lengths of subjects are shown relative to the 50th percentile (center line) as well as the 10th and 90th percentiles for a previously described reference cohort of 195 unrelated healthy individuals aged 19 to 89 years (shaded region). B, Mean O-E age-adjusted telomere length for the control reference cohort, 20 subjects without TERT mutations [−], and 20 related subjects with TERT mutations [+].*P = .035. **P = 1.4 × 10−22. IPF = idiopathic pulmonary fibrosis; LN(T/S) = circulating leukocytes and telomere lengths; O-E = observed minus expected; TERT = human gene encoding the protein component of telomerase.
), TERT mutation carriers with IPF (IPF carriers, ■), and subjects without TERT mutations (noncarriers, ○). A, The telomere lengths of subjects are shown relative to the 50th percentile (center line) as well as the 10th and 90th percentiles for a previously described reference cohort of 195 unrelated healthy individuals aged 19 to 89 years (shaded region). B, Mean O-E age-adjusted telomere length for the control reference cohort, 20 subjects without TERT mutations [−], and 20 related subjects with TERT mutations [+].*P = .035. **P = 1.4 × 10−22. IPF = idiopathic pulmonary fibrosis; LN(T/S) = circulating leukocytes and telomere lengths; O-E = observed minus expected; TERT = human gene encoding the protein component of telomerase.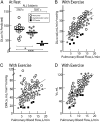
 ),and TERT mutation carriers with IPF (IPF carriers, ■). The results are graphed for all subjects. *Two-tailed Student t test P = .037 for asymptomatic carriers vs noncarriers. ***Two-tailed Student t test P = 9.1 × 10−5 for IPF carriers vs noncarriers. B-D, Recruitment of D
),and TERT mutation carriers with IPF (IPF carriers, ■). The results are graphed for all subjects. *Two-tailed Student t test P = .037 for asymptomatic carriers vs noncarriers. ***Two-tailed Student t test P = 9.1 × 10−5 for IPF carriers vs noncarriers. B-D, Recruitment of D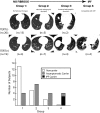


 ], respectively). A, The results are graphed for all subjects (left), those who had never smoked (middle), and those who were either current or past smokers (right). *Two-tailed Student t test P = .024. ***Two-tailed Student t test P = 7.4 × 10−4. The one-tailed Student t test P values analyzing the subgroups are 0.10 (n.s.), 0.034 (†), and 2.9 × 10−3 (†††). B, Expiratory total lung FTV vs FVC % predicted. The best-fit line is drawn (R2 = 0.256, P = .001). C, Expiratory total lung FTV vs D
], respectively). A, The results are graphed for all subjects (left), those who had never smoked (middle), and those who were either current or past smokers (right). *Two-tailed Student t test P = .024. ***Two-tailed Student t test P = 7.4 × 10−4. The one-tailed Student t test P values analyzing the subgroups are 0.10 (n.s.), 0.034 (†), and 2.9 × 10−3 (†††). B, Expiratory total lung FTV vs FVC % predicted. The best-fit line is drawn (R2 = 0.256, P = .001). C, Expiratory total lung FTV vs D
 ], respectively). A, RBC counts (millions/mL) for carriers are significantly lower than for family member control subjects. **P = 2.9 × 10−3. B, MCV (fL) is significantly higher for carriers than for noncarriers. ***P = 6.6 × 10−8. C, Platelet counts (thousands/μL) for carriers are lower than for noncarriers. *P = .032. D, RBC count (millions/μL) vs observed minus expected age-adjusted telomere length for all subjects. The best-fit line is drawn (R2 = 0.153, P = .012). E, MCV (fL) vs observed minus expected age-adjusted telomere length for all subjects. The best-fit line is drawn (R2 = 0.294, P = .0003). F, D
], respectively). A, RBC counts (millions/mL) for carriers are significantly lower than for family member control subjects. **P = 2.9 × 10−3. B, MCV (fL) is significantly higher for carriers than for noncarriers. ***P = 6.6 × 10−8. C, Platelet counts (thousands/μL) for carriers are lower than for noncarriers. *P = .032. D, RBC count (millions/μL) vs observed minus expected age-adjusted telomere length for all subjects. The best-fit line is drawn (R2 = 0.153, P = .012). E, MCV (fL) vs observed minus expected age-adjusted telomere length for all subjects. The best-fit line is drawn (R2 = 0.294, P = .0003). F, DReferences
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. - PubMed
-
- American Thoracic Society European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

