Chemotherapy-related hospitalization among community cancer center patients
- PMID: 21349949
- PMCID: PMC3228109
- DOI: 10.1634/theoncologist.2010-0354
Chemotherapy-related hospitalization among community cancer center patients
Abstract
Purpose: To describe the frequency, nature, trends, predictors, and outcomes of chemotherapy-related hospitalizations (CRHs) among a nonselected population of cancer patients treated at a community cancer center, and to explore the feasibility of implementing continuous quality improvement methodologies in routine oncology practice.
Methods: We conducted a prospective cohort study of consecutive adult cancer patients who received chemotherapy at a community cancer center January 2003 to December 2006. Demographic, comorbidity, diagnosis, treatment, and laboratory data were collected via medical record abstraction. Hospitalizations were classified as chemotherapy related or unrelated by a multidisciplinary panel. Patients who experienced CRHs were compared with those who did not. Using a randomly sampled subset of cases and controls, we built a logistic regression model to identify independent predictors of CRH.
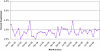
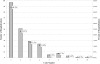
Results: Of 2,068 chemotherapy recipients, 179 (8.7%) experienced 262 CRHs. Most hospitalizations were not chemotherapy related (73.7%). The mean monthly rate of CRH was 1.5%, the median length of stay was 5 days, the most common type of CRH was gastrointestinal (46.1%) followed by infectious (31.4%), and 0.9% of chemotherapy recipients had a fatal CRH. Significant predictors of CRH included having a comorbidity score of 3-4 versus 0 and having a higher creatinine level.
Conclusions: Although the vast majority of chemotherapy recipients did not experience a CRH, these events were, unfortunately, not without serious consequences. Care should be taken when offering chemotherapy to patients with multiple comorbid conditions. Systematic efforts to monitor toxicity can lead directly to improvements in quality of care.
Conflict of interest statement
Section Editor
Section Editor
Reviewer “A” discloses a consulting/advisory relationship with Novartis and honoraria received from Novartis and Genentech. He further discloses that his spouse is employed with GlaxoSmithKline.
Reviewer “B” discloses no financial relationships.
The content of this article has been reviewed by independent peer reviewers to ensure it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
Figures
References
-
- Peppercorn JM, Weeks JC, Cook EF, et al. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. Lancet. 2004;363:263–270. - PubMed
-
- Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. - PubMed
-
- Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: An evaluation of 7 medical areas. JAMA. 2001;285:437–443. - PubMed
-
- Ladewski LA, Belknap SM, Nebeker JR, et al. Dissemination of information on potentially fatal adverse drug reactions for cancer drugs from 2000 to 2002: First results from the research on adverse drug events and reports project. [Erratum in J Clin Oncol 2004;22:1169] J Clin Oncol. 2003;21:3859–3866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

