The prenylation inhibitor manumycin A reduces the viability of Anaplasma phagocytophilum
- PMID: 21349982
- PMCID: PMC3167922
- DOI: 10.1099/jmm.0.029231-0
The prenylation inhibitor manumycin A reduces the viability of Anaplasma phagocytophilum
Abstract
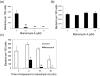
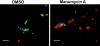
Anaplasma phagocytophilum is an obligately intracellular bacterium and is the causative agent of human granulocytic anaplasmosis (HGA), an emerging and major tick-borne disease in the USA and other parts of the world. This study showed that the prenylation inhibitor manumycin A effectively blocked A. phagocytophilum infection in host cells (HL-60 or RF/6A cells). A. phagocytophilum infection activated extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase in host cells, and manumycin A treatment reduced ERK activation in A. phagocytophilum-infected host cells. As ERK activation is required for A. phagocytophilum infection, we examined whether manumycin A inhibited the bacteria directly or through host ERK signalling. Treatment of A. phagocytophilum alone with manumycin A significantly reduced the bacterial infectivity of host cells and bacterial viability in the absence of host cells, whereas pre-treatment of host cells did not inhibit bacterial infection in host cells. The inhibitory effect of manumycin A on A. phagocytophilum infection in host cells was achieved even at a concentration 100 times lower than that required for effective inhibition of mammalian cell signalling. These results suggested that manumycin A directly inactivates the bacterium, resulting in reduced infection and ERK1/2 activation. Thus, the manumycin group of drugs may have a therapeutic potential for HGA.
Figures


References
-
- Brodasky T. F., Stroman D. W., Dietz A., Mizsak S. (1983). U-56,407, a new antibiotic related to asukamycin: isolation and characterization. J Antibiot (Tokyo) 36, 950–956 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

