Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development
- PMID: 21350016
- PMCID: PMC3050658
- DOI: 10.1242/dev.057646
Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development
Abstract
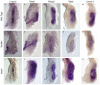
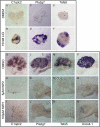
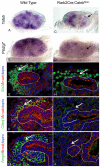
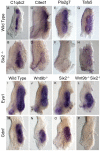
The mammalian kidney is composed of thousands of individual epithelial tubules known as nephrons. Deficits in nephron number are associated with myriad diseases ranging from complete organ failure to congenital hypertension. A balance between differentiation and maintenance of a mesenchymal progenitor cell population determines the final number of nephrons. How this balance is struck is poorly understood. Previous studies have suggested that Wnt9b/β-catenin signaling induced differentiation (mesenchymal-to-epithelial transition) in a subset of the progenitors but needed to be repressed in the remaining progenitors to keep them in the undifferentiated state. Here, we report that Wnt9b/β-catenin signaling is active in the progenitors and is required for their renewal/proliferation. Using a combination of approaches, we have revealed a mechanism through which cells receiving the same Wnt9b/β-catenin signal can respond in distinct ways (proliferate versus differentiate) depending on the cellular environment in which the signal is received. Interpretation of the signal is dependent, at least in part, on the activity of the transcription factor Six2. Six2-positive cells that receive the Wnt9b signal are maintained as progenitors whereas cells with reduced levels of Six2 are induced to differentiate by Wnt9b. Using this simple mechanism, the kidney is able to balance progenitor cell expansion and differentiation insuring proper nephron endowment. These findings provide novel insights into the molecular mechanisms that regulate progenitor cell differentiation during normal and pathological conditions.
Figures


References
-
- Barasch J., Yang J., Ware C. B., Taga T., Yoshida K., Erdjument-Bromage H., Tempst P., Parravicini E., Malach S., Aranoff T., et al. (1999). Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99, 377-386 - PubMed
-
- Boyle S., Misfeldt A., Chandler K. J., Deal K. K., Southard-Smith E. M., Mortlock D. P., Baldwin H. S., de Caestecker M. (2008). Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234-245 - PMC - PubMed
-
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264 - PubMed
-
- Carroll T. J., Park J. S., Hayashi S., Majumdar A., McMahon A. P. (2005). Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283-292 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

