Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF
- PMID: 21350188
- PMCID: PMC3119120
- DOI: 10.1152/ajpgi.00427.2010
Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF
Abstract
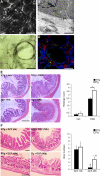
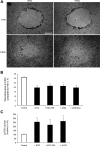
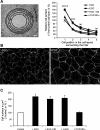
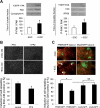
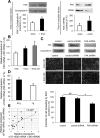
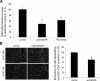
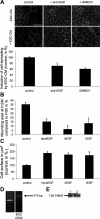
Wound healing of the gastrointestinal mucosa is essential for the maintenance of gut homeostasis and integrity. Enteric glial cells play a major role in regulating intestinal barrier function, but their role in mucosal barrier repair remains unknown. The impact of conditional ablation of enteric glia on dextran sodium sulfate (DSS)-induced mucosal damage and on healing of diclofenac-induced mucosal ulcerations was evaluated in vivo in GFAP-HSVtk transgenic mice. A mechanically induced model of intestinal wound healing was developed to study glial-induced epithelial restitution. Glial-epithelial signaling mechanisms were analyzed by using pharmacological inhibitors, neutralizing antibodies, and genetically engineered intestinal epithelial cells. Enteric glial cells were shown to be abundant in the gut mucosa, where they associate closely with intestinal epithelial cells as a distinct cell population from myofibroblasts. Conditional ablation of enteric glia worsened mucosal damage after DSS treatment and significantly delayed mucosal wound healing following diclofenac-induced small intestinal enteropathy in transgenic mice. Enteric glial cells enhanced epithelial restitution and cell spreading in vitro. These enhanced repair processes were reproduced by use of glial-conditioned media, and soluble proEGF was identified as a secreted glial mediator leading to consecutive activation of epidermal growth factor receptor and focal adhesion kinase signaling pathways in intestinal epithelial cells. Our study shows that enteric glia represent a functionally important cellular component of the intestinal epithelial barrier microenvironment and that the disruption of this cellular network attenuates the mucosal healing process.
Figures
Comment in
-
Neurogastroenterology: A role for enteric glial cells in mucosal healing.Nat Rev Gastroenterol Hepatol. 2011 May;8(5):242. doi: 10.1038/nrgastro.2011.45. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21544057 No abstract available.
Similar articles
-
Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway.Am J Physiol Gastrointest Liver Physiol. 2007 Jan;292(1):G231-41. doi: 10.1152/ajpgi.00276.2005. Epub 2006 Jan 19. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 16423922
-
Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells.Am J Physiol Gastrointest Liver Physiol. 2010 Dec;299(6):G1308-18. doi: 10.1152/ajpgi.00156.2010. Epub 2010 Aug 12. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20705905 Free PMC article.
-
Enteric glial NLRP3 inflammasome contributes to gut mucosal barrier alterations in a mouse model of diet-induced obesity.Acta Physiol (Oxf). 2025 Jan;241(1):e14232. doi: 10.1111/apha.14232. Epub 2024 Sep 17. Acta Physiol (Oxf). 2025. PMID: 39287080 Free PMC article.
-
Enteric glial cells and their role in the intestinal epithelial barrier.World J Gastroenterol. 2014 Aug 28;20(32):11273-80. doi: 10.3748/wjg.v20.i32.11273. World J Gastroenterol. 2014. PMID: 25170211 Free PMC article. Review.
-
Enteric glia.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:44-9. doi: 10.1111/j.1743-3150.2004.00474.x. Neurogastroenterol Motil. 2004. PMID: 15066004 Review.
Cited by
-
Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown.PLoS One. 2013 Jul 1;8(7):e69042. doi: 10.1371/journal.pone.0069042. Print 2013. PLoS One. 2013. PMID: 23840906 Free PMC article.
-
Semaphorin 3A controls enteric neuron connectivity and is inversely associated with synapsin 1 expression in Hirschsprung disease.Sci Rep. 2020 Sep 15;10(1):15119. doi: 10.1038/s41598-020-71865-3. Sci Rep. 2020. PMID: 32934297 Free PMC article.
-
Epithelial restitution defect in neonatal jejunum is rescued by juvenile mucosal homogenate in a pig model of intestinal ischemic injury and repair.PLoS One. 2018 Aug 23;13(8):e0200674. doi: 10.1371/journal.pone.0200674. eCollection 2018. PLoS One. 2018. PMID: 30138372 Free PMC article.
-
Role of enteric neurotransmission in host defense and protection of the gastrointestinal tract.Auton Neurosci. 2014 Apr;181:94-106. doi: 10.1016/j.autneu.2013.12.006. Epub 2013 Dec 22. Auton Neurosci. 2014. PMID: 24412639 Free PMC article. Review.
-
Enteric glia regulate Paneth cell secretion and intestinal microbial ecology.Elife. 2025 Apr 14;13:RP97144. doi: 10.7554/eLife.97144. Elife. 2025. PMID: 40227232 Free PMC article.
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53, 2006 - PubMed
-
- Amadeu TP, Seabra AB, de Oliveira MG, Costa AM. S-nitrosoglutathione-containing hydrogel accelerates rat cutaneous wound repair. J Eur Acad Dermatol Venereol 21: 629–637, 2007 - PubMed
-
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007 - PubMed
-
- Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, Tsuruo T, Beaulieu JF, Gauthier R, Vezina A, Villeneuve L, Vachon PH. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol 212: 717–728, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

