Hemorrhagic transformation of childhood arterial ischemic stroke
- PMID: 21350202
- PMCID: PMC3066279
- DOI: 10.1161/STROKEAHA.110.604199
Hemorrhagic transformation of childhood arterial ischemic stroke
Abstract
Background and purpose: The objective of this study was to describe the occurrence of hemorrhagic transformation (HT) among children with arterial ischemic stroke within 30 days after symptom onset and to describe clinical factors associated with HT.
Methods: Sixty-three children aged 1 month to 18 years with arterial ischemic stroke between January 2005 and November 2008 were identified from a single-center prospective pediatric stroke registry. All neuroimaging studies within 30 days of stroke were reviewed by a study neuroradiologist. Hemorrhage was classified according to the European Cooperative Acute Stroke Study-1 definitions. Association of HT with clinical factors, systemic anticoagulation, stroke volume, and outcome was analyzed.
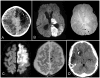
Results: HT occurred in 19 of 63 children (30%; 95% CI, 19% to 43%), only 2 (3%) of whom were symptomatic. Hemorrhage classification was hemorrhagic infarction (HI)1 in 14, HI2 in 2, parenchymal hematoma (PH)1 in 2, and PH2 in 1. HT was less common in children with vasculopathy (relative risk, 0.27; 95% CI, 0.07 to 1.06; P=0.04) than in those with other stroke mechanisms. HT was not significantly associated with anticoagulation versus antiplatelet therapy (relative risk, 0.6; 95% CI, 0.2 to 1.5; P=0.26) but was associated with larger infarct volumes (P=0.0084). In multivariable analysis, worse Pediatric Stroke Outcome Measure scores were associated with infarct volume ≥5% of total supratentorial brain volume (OR, 4.0; 95% CI, 1.1 to 15; P=0.04), and a trend existed toward association of worse Pediatric Stroke Outcome Measure scores with HT (OR, 4.0; 95% CI, 0.9 to 18; P=0.07).
Conclusions: HT occurred in 30% of children with arterial ischemic stroke within 30 days. Most hemorrhages were petechial and asymptomatic. Infarct volume was associated with HT and worse outcome.
Figures

References
-
- Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194. - PubMed
-
- Low molecular weight heparinoid, org 10172 (danaparoid), and outcome after acute ischemic stroke: A randomized controlled trial. The publications committee for the trial of org 10172 in acute stroke treatment (toast) investigators. JAMA. 1998;279:1265–1272. - PubMed
-
- The international stroke trial (ist): A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet. 1997;349:1569–1581. - PubMed
-
- Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: A double-blind randomised study. Haest study group. Heparin in acute embolic stroke trial. Lancet. 2000;355:1205–1210. - PubMed
-
- Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P, et al. Antithrombotic therapy in neonates and children: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:887S–968S. - PubMed
Publication types
MeSH terms
Grants and funding
- K23 NS062110/NS/NINDS NIH HHS/United States
- TL1RR024133/RR/NCRR NIH HHS/United States
- K23-NS062110/NS/NINDS NIH HHS/United States
- T32 NS007413/NS/NINDS NIH HHS/United States
- K23-NS52380/NS/NINDS NIH HHS/United States
- TL1 RR024133/RR/NCRR NIH HHS/United States
- K12-NS049453/NS/NINDS NIH HHS/United States
- R01 NS050488/NS/NINDS NIH HHS/United States
- R01-NS050488/NS/NINDS NIH HHS/United States
- K23 NS052380/NS/NINDS NIH HHS/United States
- K12 NS049453/NS/NINDS NIH HHS/United States
- T32-NS007413/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

