Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways
- PMID: 21350215
- PMCID: PMC3319502
- DOI: 10.1161/CIRCRESAHA.110.233775
Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways
Abstract
Rationale: A crucial step in atherogenesis is the infiltration of the subendothelial space of large arteries by monocytes where they differentiate into macrophages and transform into lipid-loaded foam cells. Macrophages are heterogeneous cells that adapt their response to environmental cytokines. Th1 cytokines promote monocyte differentiation into M1 macrophages, whereas Th2 cytokines trigger an "alternative" M2 phenotype.
Objective: We previously reported the presence of CD68(+) mannose receptor (MR)(+) M2 macrophages in human atherosclerotic plaques. However, the function of these plaque CD68(+)MR(+) macrophages is still unknown.
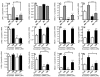
Methods and results: Histological analysis revealed that CD68(+)MR(+) macrophages locate far from the lipid core of the plaque and contain smaller lipid droplets compared to CD68(+)MR(-) macrophages. Interleukin (IL)-4-polarized CD68(+)MR(+) macrophages display a reduced capacity to handle and efflux cellular cholesterol because of low expression levels of the nuclear receptor liver x receptor (LXR)α and its target genes, ABCA1 and apolipoprotein E, attributable to the high 15-lipoxygenase activity in CD68(+)MR(+) macrophages. By contrast, CD68(+)MR(+) macrophages highly express opsonins and receptors involved in phagocytosis, resulting in high phagocytic activity. In M2 macrophages, peroxisome proliferator-activated receptor (PPAR)γ activation enhances the phagocytic but not the cholesterol trafficking pathways.
Conclusions: These data identify a distinct macrophage subpopulation with a low susceptibility to become foam cells but high phagocytic activity resulting from different regulatory activities of the PPARγ-LXRα pathways.
Figures

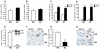

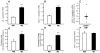

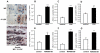
Comment in
-
Macrophage subsets in human atherosclerosis.Circ Res. 2012 Apr 27;110(9):e64; author reply e65-6. doi: 10.1161/CIRCRESAHA.112.268714. Circ Res. 2012. PMID: 22539759 No abstract available.
References
-
- Wilson HM, Barker RN, Erwig LP. Macrophages: promising targets for the treatment of atherosclerosis. Curr Vasc Pharmacol. 2009;7:234–43. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
-
- Libby P, Aikawa M, Schonbeck U. Cholesterol and atherosclerosis. Biochimica Biophysica Acta. 2000;1529:299–309. - PubMed
-
- Buhman KF, Accad M, Farese RV. Mammalian acyl-CoA:cholesterol acyltransferases. Biochimica Biophysica Acta. 2000;1529:142–154. - PubMed
-
- Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C, Duverger N, Funke H, Assman G, Dinger M, Dean M, Chimini G, Santamarina-Fojo S, Fredrickson DS, Denefle P, Brewer HB., Jr Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original tangier disease kindred. Proceedings of the National Academy of Sciences USA. 1999;96:12685–12690. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

