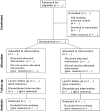
CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials
- PMID: 21350618
- PMCID: PMC3043330
- DOI: 10.4103/0976-500X.72352
CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials
Figures
References
-
- Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet. 2005;365:1159–62. - PubMed
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical