Immobilization of Rose Waste Biomass for Uptake of Pb(II) from Aqueous Solutions
- PMID: 21350666
- PMCID: PMC3042672
- DOI: 10.4061/2011/685023
Immobilization of Rose Waste Biomass for Uptake of Pb(II) from Aqueous Solutions
Abstract
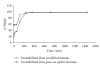
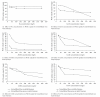
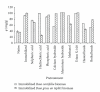
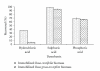
Rosa centifolia and Rosa gruss an teplitz distillation waste biomass was immobilized using sodium alginate for Pb(II) uptake from aqueous solutions under varied experimental conditions. The maximum Pb(II) adsorption occurred at pH 5. Immobilized rose waste biomasses were modified physically and chemically to enhance Pb(II) removal. The Langmuir sorption isotherm and pseudo-second-order kinetic models fitted well to the adsorption data of Pb(II) by immobilized Rosa centifolia and Rosa gruss an teplitz. The adsorbed metal is recovered by treating immobilized biomass with different chemical reagents (H(2)SO(4), HCl and H(3)PO(4)) and maximum Pb(II) recovered when treated with sulphuric acid (95.67%). The presence of cometals Na, Ca(II), Al(III), Cr(III), Cr(VI), and Cu(II), reduced Pb(II) adsorption on Rosa centifolia and Rosa gruss an teplitz waste biomass. It can be concluded from the results of the present study that rose waste can be effectively used for the uptake of Pb(II) from aqueous streams.
Figures




References
-
- Dönmez G, Aksu Z. Bioaccumulation of copper(II) and nickel(II) by the non-adapted and adapted growing CANDIDA SP. Water Research. 2001;35(6):1425–1434. - PubMed
-
- Nadeem R, Ansari TM, Khalid AM. Fourier Transform Infrared Spectroscopic characterization and optimization of Pb(II) biosorption by fish (Labeo rohita) scales. Journal of Hazardous Materials. 2008;156(1–3):64–73. - PubMed
-
- Sag Y, Ozer D, Kutsal T. A comparative study of the biosorption of lead(II) ions to Z. ramigera and R. arrhizus. Process Biochemistry. 1995;30(2):169–174.
-
- Mehta SK, Gaur JP. Use of algae for removing heavy metal ions from wastewater: progress and prospects. Critical Reviews in Biotechnology. 2005;25(3):113–152. - PubMed
-
- Basha S, Murthy ZP. Seaweeds for engineering metal biosorption: are view. In: Mason LG, editor. Focus on Hazardous Materials Research. Hauppauge, NY, USA: Nova Science; 2007. pp. 165–209.
LinkOut - more resources
Full Text Sources

