Targeting the autophagy pathway using ectopic expression of Beclin 1 in combination with rapamycin in drug-resistant v-Ha-ras-transformed NIH 3T3 cells
- PMID: 21350938
- PMCID: PMC3932692
- DOI: 10.1007/s10059-011-0034-6
Targeting the autophagy pathway using ectopic expression of Beclin 1 in combination with rapamycin in drug-resistant v-Ha-ras-transformed NIH 3T3 cells
Abstract
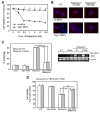
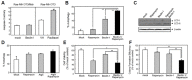
The effectiveness of an apoptosis-targeting therapy may be limited in tumor cells with defects in apoptosis. Recently, considerable attention in the field of cancer therapy has been focused on the mammalian rapamycin target (mTOR), inhibition of which results in autophagic cell death. In our study using multidrug-resistant v-Ha-ras-transformed NIH3T3 (Ras-NIH 3T3/Mdr) cells, we demonstrated that rapamycin-induced cell death may result from 2 different mechanisms. At high rapamycin concentrations (≥ 100 nM), cell death may occur via an autophagy-dependent pathway, whereas at lower concentrations (≤ 10 nM), cell death may occur after G1-phase cell cycle arrest. This effect was accompanied by upregulation of p21(Cip1) and p27(Kip1) expression via an autophagy-independent pathway. We also tested whether inhibition of mTOR with low concentrations of rapamycin and ectopic Beclin-1 expression would further sensitize multidrug resistance (MDR)-positive cancer cells by upregulating autophagy. Rapamycin at low concentrations might be insufficient to initiate autophagosome formation in autophagy but Beclin-1 overexpression triggered additional processes downstream of mTOR during G(1) cell cycle arrest by rapamycin. Our findings suggest that these combination strategies targeting autophagic cell death may yield significant benefits for cancer patients, because lowering rapamycin concentration for cancer treatment minimizes its side effects in patients undergoing chemotherapy.
Figures



References
-
- Arceci R.J., Stieglitz K., Bierer B.E. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. (1992);80:1528–1536. - PubMed
-
- Cao Y., Klionsky D.J. Physiological functions of Atg6/ Beclin 1: a unique autophagy-related protein. Cell Res. (2007);17:839–849. - PubMed
-
- Dancey J.E. Inhibitors of the mammalian target of rapamycin. Expert Opin. Investig. Drugs. (2005);14:313–328. - PubMed
-
- Daniel F., Legrand A., Pessayre D. Beclin1 mRNA strongly correlates with Bcl-XL mRNA expression in human hepatocellular carcinoma. Cancer Invest. (2007);25:226–231. - PubMed
-
- Faivre S., Kroemer G., Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. (2006);5:671–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

