Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat
- PMID: 21351151
- PMCID: PMC3596511
- DOI: 10.1002/jbmr.379
Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat
Abstract
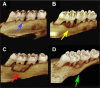
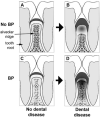
Bisphosphonates (BPs) are medications used commonly to treat primary and metastatic bone cancer, as well as osteoporosis. Although BPs improve bone mineral density, reduce fracture risk, and reduce hypercalcemia of malignancy, some patients develop BP-related osteonecrosis of the jaws (BRONJ). This devastating complication is defined as clinically exposed bone in the maxillofacial region for more than 8 weeks. Despite an increasing number of BRONJ cases since first reported, the disease pathophysiology remains largely unknown. Since published studies suggest a significant role for dental disease in the pathophysiology of BRONJ, we developed a BRONJ animal model where aggressive periodontal disease is induced by ligature placement around the crown of the right maxillary first molar in the presence of vehicle (veh) or zoledronic acid (ZA), a potent BP. Ligature placement induced significant alveolar bone loss, which was attenuated by ZA treatment. Osteonecrosis was observed associated with ligature-induced periodontitis in the ZA-treated group. This was seen as sequestration and extensive periosteal alveolar bone formation on micro-computed tomography (µCT) in the ligated site of BP-treated animals. Histologic examination confirmed these findings, seen as necrotic bone with diffuse loss of osteocytes and empty lacunae, rimming of the necrotic bone by squamous epithelium and inflammation, and exposure to the oral cavity. Importantly, the rat lesions were strikingly similar to those of BRONJ patients. Our data suggest that dental disease and potent BP therapy are sufficient for BRONJ development in the rat.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures







References
-
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. - PubMed
-
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws: 2009 update. J Oral Maxillofac Surg. 2009;67:S2–12. - PubMed
-
- Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. - PubMed
-
- Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41:351–357. - PubMed
-
- Mehrotra B, Ruggiero S. Bisphosphonate complications including osteonecrosis of the jaw. Hematology, Am Soc Hematol Educ Program. 2006;356–360:515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

