Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila
- PMID: 21351158
- PMCID: PMC3130812
- DOI: 10.1002/glia.21147
Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila
Abstract
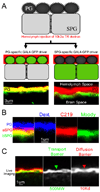
Central nervous system (CNS) physiology requires special chemical, metabolic, and cellular privileges for normal function, and blood-brain barrier (BBB) structures are the anatomic and physiologic constructs that arbitrate communication between the brain and body. In the vertebrate BBB, two primary cell types create CNS exclusion biology, a polarized vascular endothelium (VE), and a tightly associated single layer of astrocytic glia (AG). Examples of direct action by the BBB in CNS disease are constantly expanding, including key pathophysiologic roles in multiple sclerosis, stroke, and cancer. In addition, its role as a pharmacologic treatment obstacle to the brain is long standing; thus, molecular model systems that can parse BBB functions and understand the complex integration of sophisticated cellular anatomy and highly polarized chemical protection physiology are desperately needed. Compound barrier structures that use two primary cell types (i.e., functional bicellularity) are common to other humoral/CNS barrier structures. For example, invertebrates use two cell layers of glia, perineurial and subperineurial, to control chemical access to the brain, and analogous glial layers, fenestrated and pseudocartridge, to maintain the blood-eye barrier. In this article, we summarize our current understanding of brain-barrier glial anatomy in Drosophila, demonstrate the power of live imaging as a screening methodology for identifying physiologic characteristics of BBB glia, and compare the physiologies of Drosophila barrier layers to the VE/AG interface of vertebrates. We conclude that many unique BBB physiologies are conserved across phyla and suggest new methods for modeling CNS physiology and disease.
Copyright © 2011 Wiley-Liss, Inc.
Figures





References
-
- Abbott NJ. Prediction of blood-brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discovery Today: Technologies. 2004;1(4):407–416. - PubMed
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. - PubMed
-
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2010 - PubMed
-
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26(7):340–344. author reply 344-5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

