Overturning indolyne regioselectivities and synthesis of indolactam V
- PMID: 21351773
- PMCID: PMC3060274
- DOI: 10.1021/ja200437g
Overturning indolyne regioselectivities and synthesis of indolactam V
Abstract
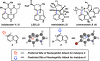
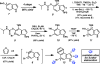
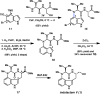
We report the design and synthesis of an indolyne that displays a reversal in regioselectivity, in both nucleophilic addition and cycloaddition reactions, compared to typical 4,5-indolynes. Our approach utilizes simple computations to predict regioselectivity in reactions of unsymmetrical arynes. With this methodology, novel benzenoid-substituted indoles can be accessed with significant regiocontrol. Furthermore, the technology provides an unconventional tactic for the synthesis of C4-substituted indole alkaloids, as demonstrated by a synthesis of indolactam V.
Figures
References
-
-
For reviews, see: Pellissier H, Santelli M. Tetrahedron. 2003;59:701–730.Wenk HH, Winkler M, Sander W. Angew. Chem. Int. Ed. 2003;42:502–528.Sanz R. Org. Prep. Proced. Int. 2008;40:217–291.Chen Y, Larock RC. Arylation Reactions Involving the Formation of Arynes. In: Ackermann L, editor. Modern Arylation Methods. Wiley–VCH; Weinheim: 2009. pp. 401–473.
-
-
-
With the exception of 3-alkoxybenzynes, most unsymmetrical arynes show modest or variable regioselectivity. For pertinent reviews, see ref . For recent examples involving naphthalynes, see: Jin T, Yamamoto Y. Angew. Chem. Int. Ed. 2007;46:3323–3325.Rémond E, Tessier A, Leroux FR, Bayardon J, Jugé S. Org. Lett. 2010;12:1568–1571. For recent examples involving 3-alkylbenzynes, see: Spiter C, Keeling S, Moses JE. Org. Lett. 2010;12:3368–3371.Crossley JA, Browne DL. Tetrahedron Lett. 2010;51:2271–2273.Hari Y, Sone R, Aoyama T. Org. Biomol. Chem. 2009;7:2804–2808.Akai S, Ikawa T, Takayanagi S.-i., Morikawa Y, Mohri S, Tsubakiyama M, Egi M, Wada Y, Kita Y. Angew. Chem. Int. Ed. 2008;47:7673–7676. For benzynocycloalkanes, see: Hamura T, Ibusuki Y, Sato K, Matsumoto T, Osamura Y, Suzuki K. Org. Lett. 2003;5:3551–3554. Scattered reports involving 3-silylbenzynes have appeared, which can react with significant regioselectivities; see: Dai M, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613–6616.Matsumoto T, Sohma T, Hatazaki S, Suzuki K. Synlett. 1993:843–846.; see also ref . For a recent example of alkoxybenzynes undergoing regioselective reactions, see: Tadross PM, Gilmore CD, Bugga P, Virgil SC, Stoltz BM. Org. Lett. 2010;12:1224–1227. see also references therein.
-
-
-
For seminal indolyne studies, see: Julia M, Huang Y, Igolen J. C. R. Acad. Sci., Ser. C. 1967;265:110–112.Igolen J, Kolb A. C. R. Acad. Sci., Ser. C. 1969;269:54–56. For related studies, see: Julia M, Goffic FL, Igolen J, Baillarge M. C. R. Acad. Sci., Ser. C. 1967;264:118–120.Julia M, Igolen J, Kolb M. C. R. Acad. Sci., Ser. C. 1971;273:1776–1777.
-
-
-
For recent indolyne studies, see: Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007–1010.Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351.Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org. Lett. 2007;9:4135–4137.Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65.Buszek KR, Brown N, Luo D. Org. Lett. 2009;11:201–204.Brown N, Luo D, Decapo JA, Buszek KR. Tetrahedron Lett. 2009;50:7113–7115.Garr AN, Luo D, Brown N, Cramer CJ, Buszek KR, VanderVelde D. Org. Lett. 2010;12:96–99.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous