Canonical WNT signaling enhances stem cell expression in the developing heart without a corresponding inhibition of cardiogenic differentiation
- PMID: 21351874
- PMCID: PMC3202895
- DOI: 10.1089/scd.2010.0490
Canonical WNT signaling enhances stem cell expression in the developing heart without a corresponding inhibition of cardiogenic differentiation
Abstract
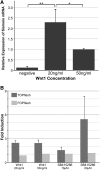
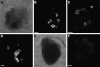
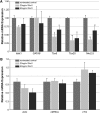
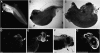
WNT signaling has been shown to influence the development of the heart. Although recent data suggested that canonical WNTs promote the emergence and expansion of cardiac progenitors in the pregastrula embryo, it has long been accepted that once gastrulation begins, canonical WNT signaling needs to be suppressed for cardiac development to proceed. Yet, this latter supposition appears to be odds with the expression of multiple canonical WNTs in the developing heart. The present study examining the effect of ectopic canonical WNT signaling on cardiogenesis in the developing frog was designed to test the hypothesis that heart formation is dependent on the inhibition of canonical WNT activity at the onset of gastrulation. Here we report that cardiac differentiation of explanted precardiac tissue from the dorsal marginal zone was not suppressed by exposure to WNT1 protein, although expression of Tbx5, Tbx20, and Nkx2.5 was selectively reduced. Pharmacological activation of WNT signaling in intact embryos using the GSK3 inhibitor SB415286 did not prevent the formation of an anatomically normal and functionally sound heart, with the only defect observed being lower levels of the cardiac transcription factor Nkx2.5. In both the explant and whole embryo studies, expression of muscle genes and proteins was unaffected by ectopic canonical WNT signaling. In contrast, canonical Wnt signaling upregulated expression of the cardiac stem cell marker c-kit and pluripotency genes Oct25 and Oct60. However, this regulatory stimulation of stem cells did not come at the expense of blocking cardiac progenitors from differentiating.
Figures

Similar articles
-
Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis.Nature. 2002 Aug 8;418(6898):636-41. doi: 10.1038/nature00921. Nature. 2002. PMID: 12167861
-
The Wnt inhibitor Dkk1 is required for maintaining the normal cardiac differentiation program in Xenopus laevis.Dev Biol. 2019 May 1;449(1):1-13. doi: 10.1016/j.ydbio.2019.02.009. Epub 2019 Feb 21. Dev Biol. 2019. PMID: 30797757 Free PMC article.
-
Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the Caspase-dependent degradation of AKT.Dev Biol. 2015 Feb 1;398(1):80-96. doi: 10.1016/j.ydbio.2014.11.015. Epub 2014 Dec 5. Dev Biol. 2015. PMID: 25482987
-
Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes.Dev Biol. 2002 Jan 15;241(2):209-28. doi: 10.1006/dbio.2001.0503. Dev Biol. 2002. PMID: 11784106 Review.
-
Wnt signaling and cardiac differentiation.Prog Mol Biol Transl Sci. 2012;111:153-74. doi: 10.1016/B978-0-12-398459-3.00007-1. Prog Mol Biol Transl Sci. 2012. PMID: 22917230 Review.
Cited by
-
Inhibition of Histone Methyltransferase, Histone Deacetylase, and β-Catenin Synergistically Enhance the Cardiac Potential of Bone Marrow Cells.Stem Cells Int. 2017;2017:3464953. doi: 10.1155/2017/3464953. Epub 2017 Jul 16. Stem Cells Int. 2017. PMID: 28791052 Free PMC article.
-
Nodal signalling in Xenopus: the role of Xnr5 in left/right asymmetry and heart development.Open Biol. 2016 Aug;6(8):150187. doi: 10.1098/rsob.150187. Open Biol. 2016. PMID: 27488374 Free PMC article.
-
Inhibition of G9a Histone Methyltransferase Converts Bone Marrow Mesenchymal Stem Cells to Cardiac Competent Progenitors.Stem Cells Int. 2015;2015:270428. doi: 10.1155/2015/270428. Epub 2015 May 21. Stem Cells Int. 2015. PMID: 26089912 Free PMC article.
-
Cardiac stem cells and their roles in myocardial infarction.Stem Cell Rev Rep. 2013 Jun;9(3):326-38. doi: 10.1007/s12015-012-9421-4. Stem Cell Rev Rep. 2013. PMID: 23238707 Review.
-
Inhibition of heart formation by lithium is an indirect result of the disruption of tissue organization within the embryo.Dev Growth Differ. 2012 Feb;54(2):153-66. doi: 10.1111/j.1440-169X.2011.01313.x. Epub 2011 Dec 12. Dev Growth Differ. 2012. PMID: 22150286 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

