Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis
- PMID: 21352489
- PMCID: PMC3070773
- DOI: 10.1111/j.1742-4658.2011.08035.x
Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis
Abstract
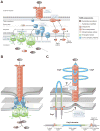
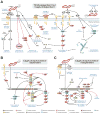
Helicobacter pylori is a very successful human-specific bacterium worldwide. Infections of the stomach with this pathogen can induce pathologies, including chronic gastritis, peptic ulcers and even gastric cancer. Highly virulent H. pylori strains encode the cytotoxin-associated gene (cag)-pathogenicity island, which expresses a type IV secretion system (T4SS). This T4SS forms a syringe-like pilus structure for the injection of virulence factors such as the CagA effector protein into host target cells. This is achieved by a number of T4SS proteins, including CagI, CagL, CagY and CagA, which by itself binds the host cell integrin member β(1) followed by delivery of CagA across the host cell membrane. A role of CagA interaction with phosphatidylserine has also been shown to be important for the injection process. After delivery, CagA becomes phosphorylated by oncogenic tyrosine kinases and mimics a host cell factor for the activation or inactivation of some specific intracellular signalling pathways. We review recent progress aiming to characterize the CagA-dependent and CagA-independent signalling capabilities of the T4SS, which include the induction of membrane dynamics, disruption of cell-cell junctions and actin-cytoskeletal rearrangements, as well as pro-inflammatory, cell cycle-related and anti-apoptotic transcriptional responses. The contribution of these signalling pathways to pathogenesis during H. pylori infections is discussed.
© 2011 The Authors Journal compilation © 2011 FEBS.
Figures

References
-
- Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–48. - PubMed
-
- Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. - PubMed
-
- Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

