Targeting of human interleukin-12B by small hairpin RNAs in xenografted psoriatic skin
- PMID: 21352568
- PMCID: PMC3058081
- DOI: 10.1186/1471-5945-11-5
Targeting of human interleukin-12B by small hairpin RNAs in xenografted psoriatic skin
Abstract
Background: Psoriasis is a chronic inflammatory skin disorder that shows as erythematous and scaly lesions. The pathogenesis of psoriasis is driven by a dysregulation of the immune system which leads to an altered cytokine production. Proinflammatory cytokines that are up-regulated in psoriasis include tumor necrosis factor alpha (TNFα), interleukin-12 (IL-12), and IL-23 for which monoclonal antibodies have already been approved for clinical use. We have previously documented the therapeutic applicability of targeting TNFα mRNA for RNA interference-mediated down-regulation by anti-TNFα small hairpin RNAs (shRNAs) delivered by lentiviral vectors to xenografted psoriatic skin. The present report aims at targeting mRNA encoding the shared p40 subunit (IL-12B) of IL-12 and IL-23 by cellular transduction with lentiviral vectors encoding anti-IL12B shRNAs.
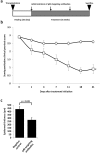
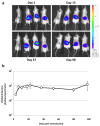
Methods: Effective anti-IL12B shRNAs are identified among a panel of shRNAs by potency measurements in cultured cells. The efficiency and persistency of lentiviral gene delivery to xenografted human skin are investigated by bioluminescence analysis of skin treated with lentiviral vectors encoding the luciferase gene. shRNA-expressing lentiviral vectors are intradermally injected in xenografted psoriatic skin and the effects of the treatment evaluated by clinical psoriasis scoring, by measurements of epidermal thickness, and IL-12B mRNA levels.
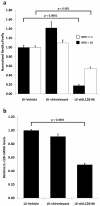
Results: Potent and persistent transgene expression following a single intradermal injection of lentiviral vectors in xenografted human skin is reported. Stable IL-12B mRNA knockdown and reduced epidermal thickness are achieved three weeks after treatment of xenografted psoriatic skin with lentivirus-encoded anti-IL12B shRNAs. These findings mimic the results obtained with anti-TNFα shRNAs but, in contrast to anti-TNFα treatment, anti-IL12B shRNAs do not ameliorate the psoriatic phenotype as evaluated by semi-quantitative clinical scoring and by immunohistological examination.
Conclusions: Our studies consolidate the properties of lentiviral vectors as a tool for potent gene delivery and for evaluation of mRNA targets for anti-inflammatory therapy. However, in contrast to local anti-TNFα treatment, the therapeutic potential of targeting IL-12B at the RNA level in psoriasis is questioned.
Figures
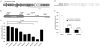

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

