Inhibition of granulocyte migration by tiotropium bromide
- PMID: 21352583
- PMCID: PMC3051905
- DOI: 10.1186/1465-9921-12-24
Inhibition of granulocyte migration by tiotropium bromide
Abstract
Study objectives: Neutrophil influx into the airways is an important mechanism in the pathophysiology of the inflammatory process in the airways of patients with chronic obstructive pulmonary disease (COPD). Previously it was shown that anticholinergic drugs reduce the release of non-neuronal paracrine mediators, which modulate inflammation in the airways. On this basis, we investigated the ability of the long-acting anticholinergic tiotropium bromide to inhibit a) alveolar macrophage (AM)-mediated chemotaxis of neutrophils, and b) cellular release of reactive oxygen species (ROS).
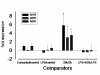
Method: AM and neutrophils were collected from 71 COPD patients. Nanomolar concentrations of tiotropium bromide were tested in AM cultured up to 20 h with LPS (1 μg/ml). AM supernatant was tested for TNFα, IL8, IL6, LTB4, GM-CSF, MIPα/β and ROS. It was further used in a 96-well chemotaxis chamber to stimulate the migration of fluorescence labelled neutrophils. Control stimulants consisted of acetylcholine (ACh), carbachol, muscarine or oxotremorine and in part PMA (phorbol myristate acetate, 0.1 μg/ml). Potential contribution of M1-3-receptors was ascertained by a) analysis of mRNA transcription by RT-PCR, and b) co-incubation with selective M-receptor inhibitors.
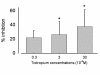
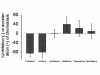
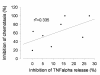
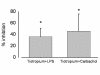
Results: Supernatant from AM stimulated with LPS induced neutrophilic migration which could be reduced by tiotropium in a dose dependent manner: 22.1 ± 10.2 (3 nM), 26.5 ± 18,4 (30 nM), and 37.8 ± 24.0 (300 nM, p < 0.001 compared to non-LPS activated AM). Concomitantly TNFα release of stimulated AM dropped by 19.2 ± 7.2% of control (p = 0.001). Tiotropium bromide did not affect cellular IL8, IL6, LTB4, GM-CSF and MIPα/β release in this setting. Tiotropium (30 nM) reduced ROS release of LPS stimulated AM by 36.1 ± 15.2% (p = 0.002) and in carbachol stimulated AM by 46.2 ± 30.2 (p < 0.001). M3R gene expression dominated over M2R and M1R. Chemotaxis inhibitory effect of tiotropium bromide was mainly driven by M3R inhibition.
Conclusion: Our data confirm that inhibiting muscarinic cholinergic receptors with tiotropium bromide reduces TNFα mediated chemotactic properties and ROS release of human AM, and thus may contribute to lessen cellular inflammation.
Figures


References
-
- Saetta M. Airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S17–S20. - PubMed
-
- Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma andnormal subjects. Am J Respir Crit Care Med. 1997;155:449–453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

