In vitro evaluation of a double-stranded self-complementary adeno-associated virus type2 vector in bone marrow stromal cells for bone healing
- PMID: 21352585
- PMCID: PMC3056728
- DOI: 10.1186/1479-0556-9-4
In vitro evaluation of a double-stranded self-complementary adeno-associated virus type2 vector in bone marrow stromal cells for bone healing
Abstract
Background: Both adenoviral and lentiviral vectors have been successfully used to induce bone repair by over-expression of human bone morphogenetic protein 2 (BMP-2) in primary rat bone marrow stromal cells in pre-clinical models of ex vivo regional gene therapy. Despite being a very efficient means of gene delivery, there are potential safety concerns that may limit the adaptation of these viral vectors for clinical use in humans. Recombinant adeno-associated viral (rAAV) vector is a promising viral vector without known pathogenicity in humans and has the potential to be an effective gene delivery vehicle to enhance bone repair. In this study, we investigated gene transfer in rat and human bone marrow stromal cells in order to evaluate the effectiveness of the self-complementary AAV vector (scAAV) system, which has higher efficiency than the single-stranded AAV vector (ssAAV) due to its unique viral genome that bypasses the rate-limiting conversion step necessary in ssAAV.
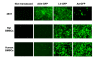
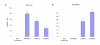
Methods: Self-complementaryAAV2 encoding GFP and BMP-2 (scAAV2-GFP and scAAV2-BMP-2) were used to transduce human and rat bone marrow stromal cells in vitro, and subsequently the levels of GFP and BMP-2 expression were assessed 48 hours after treatment. In parallel experiments, adenoviral and lentiviral vector mediated over-expression of GFP and BMP-2 were used for comparison.
Results: Our results demonstrate that the scAAV2 is not capable of inducing significant transgene expression in human and rat bone marrow stromal cells, which may be associated with its unique tropism.
Conclusions: In developing ex vivo gene therapy regimens, the ability of a vector to induce the appropriate level of transgene expression needs to be evaluated for each cell type and vector used.
Figures

References
-
- Colterjohn NR, Bednar DA. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J Bone Joint Surg Am. 1997;79:756–759. - PubMed
-
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG. et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. - PubMed
-
- Marsell R, Einhorn TA. Emerging bone healing therapies. J Orthop Trauma. pp. S4–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

