Pharmacokinetics and pharmacodynamics of artesunate and dihydroartemisinin following oral treatment in pregnant women with asymptomatic Plasmodium falciparum infections in Kinshasa DRC
- PMID: 21352601
- PMCID: PMC3056842
- DOI: 10.1186/1475-2875-10-49
Pharmacokinetics and pharmacodynamics of artesunate and dihydroartemisinin following oral treatment in pregnant women with asymptomatic Plasmodium falciparum infections in Kinshasa DRC
Abstract
Background: In many malaria-endemic countries, increasing resistance may soon compromise the efficacy of sulphadoxine-pyrimethamine (SP) for intermittent preventative treatment (IPT) of malaria in pregnancy. Artemisinin-based IPT regimens represent a promising potential alternative to SP. Pharmacokinetic and safety data supporting the use of artemisinin derivatives in pregnancy are urgently needed.
Methods: Subjects included pregnant women with asymptomatic falciparum parasitaemia between 22-26 weeks (n = 13) or 32-36 weeks gestation (n = 13), the same women at three months postpartum, and 25 non-pregnant parasitaemic controls. All subjects received 200 mg orally administered AS. Plasma total and free levels of AS and its active metabolite DHA were determined using a validated LC-MS method. Non-compartmental pharmacokinetic analysis was performed using standard methods.
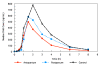
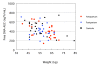
Results: All pregnant women delivered live babies. The median birth weight was 3025 grams [range 2130, 3620]; 2 of 26 babies had birth weights less than 2500 grams. Rates of parasite clearance by 12 hours post-dose were high and comparable among the groups. Rapid elimination of AS was observed in all three groups. The 90% CI for the pregnancy:postpartum ratio of geometric means for total and free AUC fell within the pre-specified 0.66 - 1.50 therapeutic equivalence interval. However, more pronounced pharmacokinetic differences were observed between the pregnancy and control subjects, with the 90% CI for the pregnancy:control ratio of geometric means for both total 0.68 (90% CI 0.57-0.81) and free AUC 0.78 (90% CI 0.63-0.95) not fully contained within the 0.66 - 1.50 interval. All subjects cleared parasites rapidly, and there was no difference in the percentage of women who were parasitaemic 12 hours after dosing.
Conclusions: A single dose of orally administered AS was found to be both effective and without adverse effects in this study of second and third trimester pregnant women in the DRC. Although DHA AUC during pregnancy and postpartum were similar, the AUC for the pregnant group was less than the non-pregnant controls. The findings of this study suggest that additional studies on the pharmacokinetics of AS in pregnant women are needed.
Trial registration: ClinicalTrials.gov: NCT00538382.
Figures
References
-
- World Health Organization. World Malaria Report 2009. Geneva, Switzerland: World Health Organization; 2009.
-
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1-2 Suppl):28–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials