Effects of currently prescribed LDL-C-lowering drugs on PCSK9 and implications for the next generation of LDL-C-lowering agents
- PMID: 21352602
- PMCID: PMC3055818
- DOI: 10.1186/1476-511X-10-38
Effects of currently prescribed LDL-C-lowering drugs on PCSK9 and implications for the next generation of LDL-C-lowering agents
Abstract
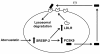
Background: During the past decade, proprotein convertase subtilisin kexin type 9 (PCSK9) has been identified as a key regulator of serum LDL-cholesterol (LDL-C) levels. PCSK9 is secreted by the liver into the plasma and binds the hepatic LDL receptor, causing its subsequent degradation. In humans, gain-of-function mutations in PCSK9 cause a form of familial hypercholesterolemia that manifests with dramatically increased serum levels of LDL-C, while loss-of-function mutations in PCSK9 are associated with significantly decreased LDL-C and cardiovascular risk.
Results: Initial studies in animals and cultured cells demonstrated that statins increased PCSK9 mRNA expression, resulting in many research groups exploring the effect of statins on PCSK9 levels in humans. We first reported that statins increased human PCSK9 circulating protein levels. Additional researchers subsequently confirmed these observations, further prompting many laboratories including our own to examine the effect of other lipid lowering medications on PCSK9 levels. Our observation that fenofibrate (200 mg/day) significantly increased PCSK9 levels was confirmed by another laboratory, and an additional group demonstrated that ezetimibe also increased PCSK9 levels.
Conclusions: It has become clear that the major classes of commonly prescribed lipid-lowering medications increase serum PCSK9 levels. These observations almost certainly explain why these agents are not more effective in lowering LDL-C and suggest that efforts should be made toward the development of new LDL-C lowering medications that either do not increase circulating PCSK9 levels or work through decreasing or inhibiting PCSK9.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

