Excitation-transcription coupling in sympathetic neurons and the molecular mechanism of its initiation
- PMID: 21352861
- PMCID: PMC3930329
- DOI: 10.1016/j.neures.2011.02.004
Excitation-transcription coupling in sympathetic neurons and the molecular mechanism of its initiation
Abstract
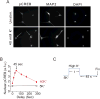
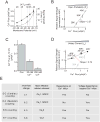
In excitable cells, membrane depolarization and activation of voltage-gated Ca²+ (Ca(V)) channels trigger numerous cellular responses, including muscle contraction, secretion, and gene expression. Yet, while the mechanisms underlying excitation-contraction and excitation-secretion coupling have been extensively characterized, how neuronal activity is coupled to gene expression has remained more elusive. In this article, we will discuss recent progress toward understanding the relationship between patterns of channel activity driven by membrane depolarization and activation of the nuclear transcription factor CREB. We show that signaling strength is steeply dependent on membrane depolarization and is more sensitive to the open probability of Ca(V) channels than the Ca²+ entry itself. Furthermore, our data indicate that by decoding Ca(V) channel activity, CaMKII (a Ca²+/calmodulin-dependent protein kinase) links membrane excitation to activation of CREB in the nucleus. Together, these results revealed some interesting and unexpected similarities between excitation-transcription coupling and other forms of excitation-response coupling.
Copyright © 2011 Elsevier Ireland Ltd and the Japan Neuroscience Society. All rights reserved.
Figures



References
-
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. - PubMed
-
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N'-tetracetic acid. Biochimica et biophysica acta. 1972;267:605–608. - PubMed
-
- Augustine GJ. How does calcium trigger neurotransmitter release? Current opinion in neurobiology. 2001;11:320–326. - PubMed
-
- Beam KG, Franzini-Armstrong C. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol. 1997;52:283–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

