Long-term safety and serologic response to measles, mumps, and rubella vaccination in HIV-1 infected adults
- PMID: 21352938
- PMCID: PMC3073409
- DOI: 10.1016/j.vaccine.2011.02.013
Long-term safety and serologic response to measles, mumps, and rubella vaccination in HIV-1 infected adults
Abstract
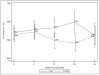
We analyzed HIV viral load (VL) and CD4 count changes, and antibody responses following MMR vaccination of individuals in the U.S. Military HIV Natural History Study cohort. Cases receiving at least one dose of MMR vaccine after HIV diagnosis were matched 1:2 to HIV-positive controls not receiving the vaccine. Baseline was defined as time of vaccination for cases and indexed and matched to the time post-HIV diagnosis for controls. Changes in CD4 count and VL at 6, 12, 18 and 24 months were compared between cases and controls using a general linear model. Available sera from cases were tested for MMR seropositivity at baseline and post-vaccination at 6, 12, 18, and 24 months. Overall mean CD4 count change from baseline through 24 months was 20 (±23) cells/μL greater for cases than controls (p=0.39). Similar non-significant changes in CD4 cell count were seen in the subset of those not on HAART at baseline. VL changes were small and similar between groups (mean differential change -0.04 (±0.18) log(10) copies/mL; p=0.84). Of 21 vaccinated participants with baseline serologic testing, 14 (67%) were reactive to measles, 19 (91%) to mumps, and 20 (95%) to rubella. Three (43%) of 7 participants nonreactive to measles developed measles IgG; for mumps, 1 (50%) of 2 developed mumps IgG; for rubella, 1 (100%) developed rubella IgG. MMR vaccination did not result in detrimental immunologic or virologic changes through 24 months post-vaccination.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Seroprevalence of antibodies to measles, mumps, and rubella, and serologic responses after vaccination among human immunodeficiency virus (HIV)-1 infected adults in Northern Thailand.BMC Infect Dis. 2016 Apr 30;16:190. doi: 10.1186/s12879-016-1499-x. BMC Infect Dis. 2016. PMID: 27138005 Free PMC article.
-
Serological status of measles, mumps, and rubella antibodies in HIV-positive women of childbearing age at a referral hospital in Iran.AIDS Res Ther. 2025 Apr 3;22(1):42. doi: 10.1186/s12981-025-00735-7. AIDS Res Ther. 2025. PMID: 40181371 Free PMC article.
-
[Antibody persistence following on different vaccination strategies of domestic measles, mumps and rubella combined attenuated live vaccine: a 3-year follow-up study].Zhonghua Yu Fang Yi Xue Za Zhi. 2017 Apr 6;51(4):336-340. doi: 10.3760/cma.j.issn.0253-9624.2017.04.011. Zhonghua Yu Fang Yi Xue Za Zhi. 2017. PMID: 28395468 Chinese.
-
Vaccines for measles, mumps, rubella, and varicella in children.Cochrane Database Syst Rev. 2020 Apr 20;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Nov 22;11:CD004407. doi: 10.1002/14651858.CD004407.pub5. PMID: 32309885 Free PMC article. Updated.
-
Successes and challenges for preventing measles, mumps and rubella by vaccination.Curr Opin Virol. 2019 Feb;34:110-116. doi: 10.1016/j.coviro.2019.01.002. Epub 2019 Mar 7. Curr Opin Virol. 2019. PMID: 30852425 Review.
Cited by
-
Measles, mumps, rubella and VZV: importance of serological testing of vaccine-preventable diseases in young adults living with HIV in Germany.Epidemiol Infect. 2017 Jan;145(2):236-244. doi: 10.1017/S095026881600217X. Epub 2016 Oct 26. Epidemiol Infect. 2017. PMID: 27780480 Free PMC article.
-
Research priorities for global measles and rubella control and eradication.Vaccine. 2012 Jul 6;30(32):4709-16. doi: 10.1016/j.vaccine.2012.04.058. Epub 2012 Apr 28. Vaccine. 2012. PMID: 22549089 Free PMC article.
-
Protection of Vaccine Preventable Diseases in a Population of HIV-Infected Children: A 3 Years Prospective Study.Vaccines (Basel). 2021 Nov 15;9(11):1331. doi: 10.3390/vaccines9111331. Vaccines (Basel). 2021. PMID: 34835262 Free PMC article.
-
Vaccinations for the HIV-Infected Adult: A Review of the Current Recommendations, Part II.Infect Dis Ther. 2017 Sep;6(3):333-361. doi: 10.1007/s40121-017-0165-y. Epub 2017 Aug 5. Infect Dis Ther. 2017. PMID: 28780736 Free PMC article. Review.
-
Seroprevalence of antibodies to measles, mumps, and rubella, and serologic responses after vaccination among human immunodeficiency virus (HIV)-1 infected adults in Northern Thailand.BMC Infect Dis. 2016 Apr 30;16:190. doi: 10.1186/s12879-016-1499-x. BMC Infect Dis. 2016. PMID: 27138005 Free PMC article.
References
-
- Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-3):1–12. - PubMed
-
- Chen TH, Kutty P, Lowe LE, Hunt EA, Blostein J, Espinoza R, et al. Measles outbreak associated with an international youth sporting event in the United States, 2007. Pediatr Infect Dis J. 2010;29(9):794–800. - PubMed
-
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358(15):1580–9. - PubMed
-
- Centers for Disease Control and Prevention. Brief Update: Mumps Outbreak--New York and New Jersey, June 2009-January 2010. MMWR. 2010;59(5):125–29. - PubMed
-
- Centers for Disease Control and Prevention. Recommended adult immunization scheduled--United States, 2009. MMWR. 2009;57:Q1–Q4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials