A comparative analysis of HIV-specific mucosal/systemic T cell immunity and avidity following rDNA/rFPV and poxvirus-poxvirus prime boost immunisations
- PMID: 21352941
- PMCID: PMC3244379
- DOI: 10.1016/j.vaccine.2011.01.106
A comparative analysis of HIV-specific mucosal/systemic T cell immunity and avidity following rDNA/rFPV and poxvirus-poxvirus prime boost immunisations
Abstract
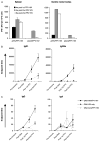
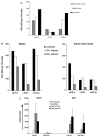
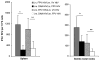
In this study we have firstly compared a range of recombinant DNA poxvirus prime-boost immunisation strategies and shown that combined intramuscular (i.m.) 2× DNA-HIV/intranasal (i.n.) 2× FPV-HIV prime-boost immunisation can generate high-level of HIV-specific systemic (spleen) and mucosal (genito-rectal nodes, vaginal tissues and lung tissues) T cell responses and HIV-1 p24 Gag-specific serum IgG1, IgG2a and mucosal IgG, SIgA responses in vaginal secretions in BALB/c mice. Data indicate that following rDNA priming, two rFPV booster immunisations were necessary to generate good antibody and mucosal T cell immunity. This data also revealed that mucosal uptake of recombinant fowl pox (rFPV) was far superior to plasmid DNA. To further evaluate CD8+ T cell immunity, i.m. 2× DNA-HIV/i.n. 1× FPV-HIV immunisation strategy was directly compared with single shot poxvirus/poxvirus, i.n. FPV-HIV/i.m. VV-HIV immunisation. Results indicate that the latter strategy was able to generate strong sustained HIV-specific CD8+ T cells with higher avidity, broader cytokine/chemokine profiles and better protection following influenza-K(d)Gag(197-205) challenge compared to rDNA poxvirus prime-boost strategy. Our findings further substantiate the importance of vector selection/combination, order and route of delivery when designing effective vaccines for HIV-1.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
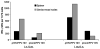


References
-
- Kelleher AD, Puls RL, Bebbington M, Boyle D, Ffrench R, Kent SJ, et al. A randomised, placebo-controlled phase I trial of DNA prime, recombinant fowlpox virus boost prophylactic vaccine for HIV-1. AIDS. 2006;20(2):294–7. - PubMed
-
- Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–11. - PubMed
-
- Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr Mol Med. 2003;3(3):217–28. - PubMed
-
- Stevceva L, Strober W. Mucosal HIV vaccines: where are we now? Curr HIV Res. 2004;2(1):1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

